-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 751-755
doi:10.5923/j.ajmms.20231305.43
Received: May 12, 2023; Accepted: May 23, 2023; Published: May 31, 2023

Determination of M. Tuberculosis Sensitivity to Second-Line Anti-Tuberculosis Drugs Using Phenotypic XDR Test
Sayfutdinov Zainiddin Asamudinovich
Republican Specialized Scientific-Practical Medical Center for Phthisiology and Pulmonology, Tashkent, Uzbekistan
Correspondence to: Sayfutdinov Zainiddin Asamudinovich, Republican Specialized Scientific-Practical Medical Center for Phthisiology and Pulmonology, Tashkent, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

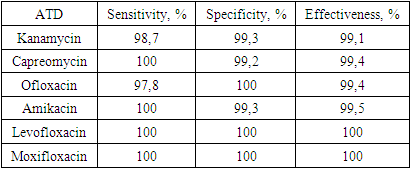
The objective of the study: to evaluate the phenotypic XDR test for susceptibility testing of M. tuberculosis to second line anti-tuberculosis drugs in clinical trials and as part of annual professional testing cycles. Cultures of M. tuberculosis (n = 90) freshly isolated on egg media from clinical samples collected in tuberculosis patients were tested using the Bactec MGIT 960 system and the XDR test under identical conditions. Well-studied strains of M. tuberculosis (n = 216) were repeatedly cultured on Middlebrook 7H10 medium before the study. The drug susceptibility of the cultures was assessed using the XDR test by the nitrate reductase method. A high concurrence (96.7-100%) of the results was shown when testing susceptibility of 90 M. tuberculosis isolates to kanamycin, amikacin, capreomycin and ofloxacin using the XDR test and the Bactec MGIT 960 system with comparable test periods. The use of the XDR test for drug susceptibility testing of 216 M. tuberculosis strains in eleven annual professional testing cycles provided the results consistent with the consensus one for kanamycin, capreomycin, ofloxacin and amikacin in 98.6, 99.4, 99.4, and 99.0% of cases, respectively. For moxifloxacin and levofloxacin additionally incorporated to the XDR test, completely identical results were obtained.
Keywords: M. tuberculosis, Drug susceptibility, Second line anti-tuberculosis drugs, Nitrate reductase method, the XDR test
Cite this paper: Sayfutdinov Zainiddin Asamudinovich, Determination of M. Tuberculosis Sensitivity to Second-Line Anti-Tuberculosis Drugs Using Phenotypic XDR Test, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 751-755. doi: 10.5923/j.ajmms.20231305.43.
1. Introduction
- Morbidity and mortality from tuberculosis (TB) are still high worldwide and in Russia, despite the downward trend [4,7]. Tuberculosis with multiple (MDR-TB) and widespread drug resistance (XDR-TB, or XDR-TB) is a serious problem. The World Health Organization (WHO) estimates that XDR-TB averages 6% among MDR-TB [7,9]. The main factors of the high prevalence of drug-resistant TB are the insufficient level of laboratory diagnostics and empirical treatment of patients infected with resistant strains of M. tuberculosis, without drug sensitivity testing [5,13,15]. Therefore, the use of fast and reliable diagnostic tools with a simple experimental protocol can help reduce the prevalence of drug-resistant TB.Classical methods for determining the drug sensitivity of M. tuberculosis are quite long and require 3-4 weeks. to get the results. The automated Bactec 960 MGIT system, molecular genetic tests (biochips, Xpert MTB/ RIF, LPA, etc.), tests based on the use of mycobacteriophages significantly accelerate the determination of drug resistance, but require expensive equipment, or are limited to a small number of tested determinants of resistance and genomic regions, or are at the stage of scientific development [1,3,8,10,12]. The costs and problems of interpreting the data of complete genomic sequencing (WGS) currently do not allow its widespread use in clinical practice [6,16,18].The nitrate reductase method (NPM) has been approved by WHO as a cost-effective and fast method for determining the drug sensitivity of M. tuberculosis [14,19]. The HPM principle is used in working with a TB test kit for accelerated identification and determination of drug sensitivity to anti-tuberculosis drugs of the 1st series, which reduces the time to obtain results by almost 3 times compared to the absolute concentration method [2,11,20].The purpose of the study: evaluation of the phenotypic XDR test in determining the sensitivity of M. Tuberculosis to anti-tuberculosis drugs (ATD) of the 2nd series in clinical trials and as part of annual professional testing cycles.
2. Materials and Methods
- Bioethical requirements. The clinical strains used in the work do not contain personal data of patients, they are marked without specifying the surname, date of birth, residential address, medical history number, personal documents and other personal materials. In accordance with the requirements of the bioethical committee, each patient, upon admission to the clinic, concluded an agreement with the medical institution containing consent to treatment and laboratory examination.The tests examined 90 freshly isolated M.Tuberculosis isolates from clinical samples (sputum, bronchial lavage) obtained from newly identified and previously treated patients with pulmonary tuberculosis (TB), and 216 well-characterized M. Tuberculosis strains in the National Reference Laboratory at the Republican Scientific and Practical Medical Center of Phthisiology and Pulmonology. M.tuberculosis isolates (n = 90) were used directly from the isolation medium (Levenstein-Jensen medium) after 2-3 weeks. after the appearance of visible growth without additional storage and subcultivation. M. Tuberculosis strains were subcultivated on Middlebrook medium 7H10 before the study and incubated for 2-3 weeks.A method for determining the drug sensitivity of M.Tuberculosis using an XDR test. Bacterial suspensions were prepared by suspending M. tuberculosis isolates in 0.3 ml of 0.2% sterile twin-80 solution on Vortex with glass beads with a diameter of 1 mm. The turbidity of the suspensions was adjusted to 5 units according to the standard sample of turbidity of the CCA-42-28-86 P and then diluted 1:10 with a sterile 0.9% sodium chloride solution. 0.2 ml of the resulting suspension was inoculated into each vial of XDR test using a syringe by piercing a rubber stopper. The seeded vials were placed in an inclined position in a packing box, which was then placed in a thermostat at a temperature of (36 ± 1)°C. After 8 days of incubation, 0.5 ml of 7.5% Griss reagent solution was added to one of the two control vials. If an intense pink or red color appeared in it (at least 3+ on the color scale), 0.5 ml of 7.5% Griss reagent solution was added to all remaining vials and visual accounting of the results on the appearance of the color was carried out. If the color in the control vial is less than 3 +, this vial was destroyed, the rest continued to incubate for up to 10-12 days, then a solution of Griss reagent was introduced into the remaining vials and the results were recorded for the presence of color. The culture was considered sensitive to ATD if there was no appearance of coloration in the vial with it (with intense coloration in the control vial). The culture was considered stable when coloring appeared (from 1+ to 5+).When analyzing the results, the diagnostic characteristics of the phenotypic XDR test were evaluated: sensitivity (the ability to determine true drug resistance), specificity (the ability to determine true drug sensitivity), effectiveness (the proportion of correct results to the total number of results). The proportion of the number of matching resistant/sensitive strains determined by the phenotypic XDR test and the reference method to the total number of resistant/sensitive strains identified by the reference method was taken as true drug sensitivity/resistance. Diagnostic characteristics are defined for each ATD separately. For strains with inconsistent results in cycles 16 and 20, the corresponding genes were sequenced by Sanger.
3. Result
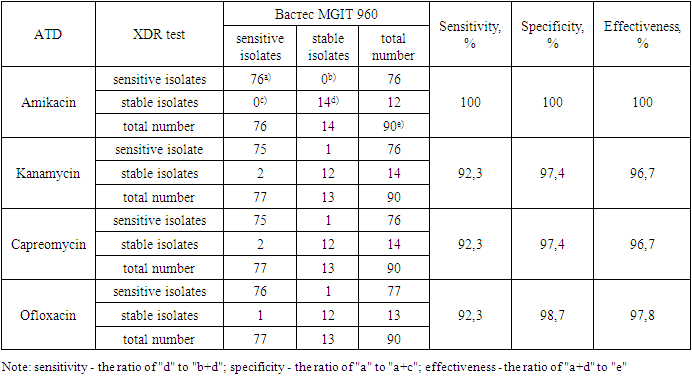
- The phenotypic XDR test is a set of ready-to-use nutrient media containing anti-tuberculosis drugs: 1 mcg/ml isoniazid, 40 mcg/ml rifampicin, 30 mcg/ml kanamycin, 30 mcg/ml amikacin, 30 mcg/ml capreomycin and 3 mcg/ml ofloxacin and without drugs (control). The nutrient media for determining sensitivity to isoniazid and rifampicin included in the XDR test are similar to the media included in the TB test kit. In this article, the results of determining the sensitivity of M. Tuberculosis to ATD of the 1st series are not given.Clinical studies of the phenotypic XDR test were conducted in the National Reference Laboratory at the Republican Scientific and Practical Medical Center of Phthisiology and Pulmonology. During the tests, 90 isolates with different drug sensitivity spectra were tested. The percentage of coincidence (effectiveness) of the results obtained using the phenotypic XDR test and Vastes MGIT 960 for all four anti-tuberculosis drugs studied is quite high and amounts to 96.7% for kanamycin and capreomycin, 97.8% for ofloxacin and 100% for amikacin. The sensitivity and specificity of the phenotypic XDR test also have high values: 100 and 100% for amikacin, 92.3 and 97.4% for kanamycin and capreomycin, 92.3 and 98.7% for ofloxacin. The time to obtain results using the phenotypic XDR test is 9.5 days (range 8-14) and for MGIT 960 Vastes - 6.8 days (range 4-9) (table 1).
|
|
4. Conclusions
- In the course of comparative studies of the phenotypic XDR test and the automated Bactec MGIT 960 system, a high coincidence of results was shown in determining the sensitivity of 90 M. Tuberculosis isolates to kanamycin, amikacin, capreomycin and ofloxacin. The timing of determining the drug sensitivity of MBT using a phenotypic XDR test is comparable to the timing of determination on Bactec MGIT 960.When using a phenotypic XDR test to determine the drug sensitivity of 216 M. Tuberculosis strains during 11 annual cycles of professional testing, the results were obtained that coincide with the consensus for kanamycin, capreomycin, ofloxacin and amikacin in 98.6; 99.4; 99.4; 99.0% of cases, respectively.For moxifloxacin and levofloxacin, which were additionally introduced into the phenotypic XDR test, completely identical results were obtained in two cycles. The positive results and the versatility of the production technology show the fundamental possibility of modifying the phenotypic XDR test by changing the list of ATD in accordance with the requirements of modern standards. To introduce the modified phenotypic XDR test into production, additional clinical trials will be conducted using M. Tuberculosis isolates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML