-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 743-746
doi:10.5923/j.ajmms.20231305.41
Received: May 15, 2023; Accepted: May 26, 2023; Published: May 27, 2023

Early Diagnosis of Renal Dysfunction in Patients with Chronic Heart Failure who have had and have not had Covid-19
Abdigaffar G. Gadaev, Nigora V. Pirmatova, Matluba E. Rakhimova
Tashkent Medical Academy, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article discusses early diagnosis of renal dysfunction in patients with chronic heart failure who have had and have not had Covid-19.
Keywords: Diagnosis, Renal dysfunction, Patients with chronic heart failure, Covid-19
Cite this paper: Abdigaffar G. Gadaev, Nigora V. Pirmatova, Matluba E. Rakhimova, Early Diagnosis of Renal Dysfunction in Patients with Chronic Heart Failure who have had and have not had Covid-19, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 743-746. doi: 10.5923/j.ajmms.20231305.41.
1. Introduction
- As it is known, chronic heart failure (CHF) is one of the vulnerable problems of modern cardiology. The prevalence of CHF according to population studies in countries around the world varies from 0.3% to 5.3%. According to the Framingham Heart Study, the lifetime risk of heart failure in men is 21% and 20% in women [5]. In the United States alone, the proportion of deaths due to chronic heart failure has increased from 5.8% to 9.9% over 20 years [8].The work of the heart and blood circulation in the body is controlled by vascular tone and blood volume, the balance of which is provided by the kidneys. The kidneys, in turn, being an organ involved in important metabolic, humoral processes, are subject to acute and chronic effects in various cardiovascular diseases (CVD), including CHF, and affect their formation and progression. Renal dysfunction is associated with a higher recurrence rate of myocardial ischemia, myocardial infarction, serious hemorrhagic complications, acute heart failure, atrial and ventricular fibrillation. Even a slight decrease in kidney function leads to a deterioration in the course of the main cardiological pathology, the occurrence of complications of which - a decrease in the contractile function of the myocardium, in turn, affects the work of the kidneys in the most negative way.Traditionally, kidney dysfunction is usually determined by such indicators as blood creatinine, micro and macroalbuminuria, cystatin, glomerular filtration rate (GFR). Recently, scientists have begun to study the functional and anatomical structure of the renal nephron in more depth, in order to identify even earlier markers of renal dysfunction. In this connection, our study was aimed at identifying renal dysfunction in its early stages, before microalbuminuria starts. Purpose: to identify markers of early kidney damage - renal dysfunction in CHF patients with systolic dysfunction.
2. Materials and Methods
- The study included 225 patients with chronic heart failure II-III FC according to the classification of the New York Heart Association (NYHA). Group I consist of 165 patients, which are patients with CHF who have had Covid-19, and group II - 60 patients with CHF who have not had Covid-19. The average age of patients in group I was 64.03±0.8, and in group II - 64.5±3.4 years. In the first group of patients with CHF, men were 98 (59.4%), and women 67 (40.6%), in the second group, there were 37 (61.7%) men, 23 (38.3%) women. The average duration of the disease from the anamnesis was 5.3±0.5 years in the first group, 4.2±0.3 years in the second.According to the functional class, CHF patients were divided into 2 groups. In the first group - with II FC - 35 (21.2%), with III FC - 130 (78.8%) patients, in the second group - with II FC - 18 (30%), with III FC - 42 (70%) patients. From the anamnesis, the duration of the disease was 5.3+0.5 years in group I, and 4.2 years in group II. With CHF II FC in group I there were 35 (21.2%), in group II - 18 (30%) patients, with CHF III FC in group I there were 130 (78.8%), and in group II 42 (70%) of patients.According to the structure of comorbidity, the following changes were noted. Anemia was detected in group I in 109 (66%) cases and in group II in 35 (58%) patients, respectively. In group I - 105 (63.6%) patients had a history of myocardial infarction, and in group II - in 36 (60%). Coronary artery bypass grafting and stenting were performed in 45 (27.3%) patients of group I, and in 19 (31.6%) patients from group II. Various types of arrhythmias were detected in 51 (30.9%) patients of group I, and in 11 (18.3%) patients of group II. In 59 (37.75%) patients of group I, obesity was detected, in group II - in 15 (25%), in both groups, 8% of patients were diagnosed with COPD.All patients underwent echocardiographic examination using the SONOSCAPE S20 (China) equipment. Patients with systolic dysfunction were selected, i.e. ejection fraction less than 50% according to Simpson.Immunoenzymatic assay of Cystatin C, TNF-α, were carried out on a Rayto analyzer using Vector Best (Russia), Collagen Type IV α1 from Elabscience (America). A biochemical blood test was carried out using a Midray BA-88A biochemical analyzer. Blood creatinine was checked using the reagent of the German company “Human” (Germany) according to the Jaffe method.Daily microalbuminuria was determined using the benzethonium chloride reagent by the colorimetric method on a photometer with an optical density of 430 nm.Nephrine was determined in the morning portion of urine using an enzyme immunoassay kit (ELISA Kit) manufactured by CUSABIO (China) with the calculation of the concentration per unit of urine creatinine in the test sample.Cystatin C in blood serum was determined using a DiaSys Diagnostic Systems kit (Germany).
3. Results
- The filtration function of the kidneys, as noted above, was traditionally assessed by the level of endogenous creatinine. In the study of a biochemical blood test, the creatinine level in the examined patients was 114.3±3.3 µmol/l (p<0.001) in the first group, and in the group of patients who did not have Covid-19, it was 94.7±3.2 µmol / l. As many parameters are known to affect the level of creatinine, it was therefore assumed that cystatin-C is a more reliable indicator of renal dysfunction, the content of which, unlike creatinine, is not affected by gender, age, body weight and muscle mass, and nutritional characteristics.
|
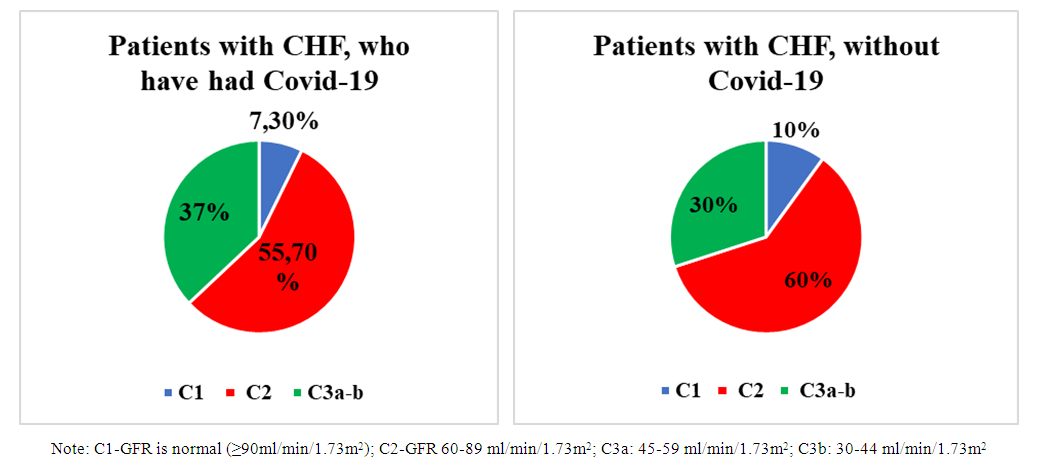 | Figure 1. Distribution of patients by stages based on the level of glomerular filtration rate |
|
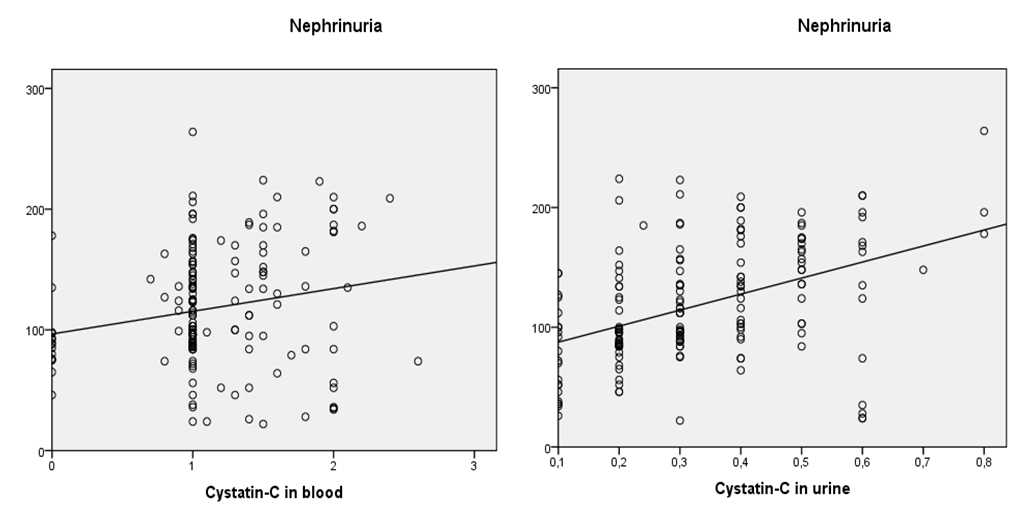 | Figure 2. Correlation graph of cystatin C in blood and urine with nephrinuria |
4. Conclusions
- Based on the study, a violation of glomerular filtration was revealed, an increase in blood creatinine was detected, a decrease in GFR was noted in both groups, a greater increase in the content of cystatin-C in group I than in group II, and in the same group a significant decrease in GFR, which is due, possibly, to the direct effect of the virus on podocytes, which further worsened glomerular filtration.In groups I and II of patients, the level of nephrin in the urine was 98.7±3.67 ng/ml (p<0.001), in group II - 68.9±3.0 ng/ml, which is significantly higher than the reference values, and this proves damage to the podocyte structure.When studying the correlation, a strong positive relationship of nephrinuria with urine cystatin, as well as with blood cystatin, was revealed, which once again confirms the primary damage to the podocytic link of the renal glomerulus.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML