Nortaeva Nilufar Abdurakhimovna1, Akhmedova Sayyora Mukhamadovna2, Nortaev Azamat Bekmatovich1, Berdiev Otabek Vakhobovich1
1Assistant, Tashkent Medical Academy, Uzbekistan
2Doctor of Medical Sciences, Professor, Tashkent Medical Academy, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article provides information on age–related changes in the morphological and morphometric indicators of the dentition system of school–aged children with hypothyroidism. Changes in children between the ages of 7 and 16 have been studied. Morphological changes of tooth enamel, cementum, dentine, and pulp were studied. Morphometric changes were mainly carried out using the Bunak method. We used only parameter 5a from the Bunak method. That is, the physiognomic height of the face, the total morphological height of the face, the morphological width of the face, the upper depth of the face, the height of the mandibular body. The specificity of these indicators in each age group was studied.
Keywords:
Children, Morphometric indicators, Morphological changes, Teeth and jaws, Anthropometric measurements
Cite this paper: Nortaeva Nilufar Abdurakhimovna, Akhmedova Sayyora Mukhamadovna, Nortaev Azamat Bekmatovich, Berdiev Otabek Vakhobovich, Morphological and Morphometric Indicators of the Dentition System of School–Aged Children with Hypothyroidism, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 717-720. doi: 10.5923/j.ajmms.20231305.36.
1. Introduction
Symptoms of hypothyroidism include damage to the hard tissues of the teeth and changes in the composition of saliva [13,14]. Thyroid hormone deficiency is accompanied by metabolic changes that cause dental caries [11,12]. The analysis of the literature showed that there are scientific data on the changes in the teeth and jaw bones caused by the lack of thyroid hormones [6,8,10]. However, these data are incomplete and the changes observed in the teeth are not sufficiently explained. In this regard, it is necessary to constantly give iodine preparations to children not only in the mother’s womb, but also at school age and during adolescence [2,5,7,9]. This helps children grow and develop normally and improves mental activity of children [1,3,4,14].The purpose of the study. Calculation and study of morphological and morphometric indicators in the tooth–jaw system of school–aged children with hypothyroidism.
2. Research Materials and Methods
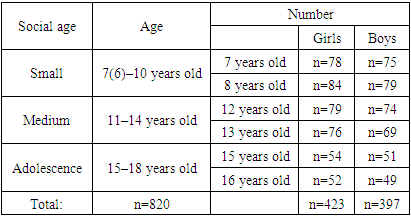
As an object of research, it was conducted in the clinical bases of the 59th polyclinic of the Yakkasaroy district of the city of Tashkent and the 1st polyclinic of the Yangiyol district of the Tashkent region, following all the necessary ethical and deontological norms. We selected 260 school–age children living in Tashkent city and Yangiyol district of Tashkent region. Bunak’s method was used to measure the anthropometric parameters of the teeth–jaw system in all the selected children. The youngest of the children is 8 years old, and the oldest is 16 years old. Therefore, we divided the children from 8 to 16 years into 3 groups. In this, age periodization based on widespread social principles in our country was used (Table 1).Table 1. According to the status of school–aged children
 |
| |
|
820 children of both sexes between the ages of 7 and 16 living permanently in the Tashkent region were involved. Of the total number of practically healthy children contingent (n=820), 397 (48.4±1.7%) are boys and 423 (51.6±1.7%) are girls. Distribution of children by place of residence showed the following result: 375 (45.7±1.6%) children living in rural areas; there are 445 (54.3±1.6%) children in the city.An important parameter for evaluating a child’s physical development is the upbringing of the child in organized groups. In this regard, we decided to divide the children involved in the study according to the place of education and upbringing.Based on age classification, the first group is 8–9 years old school children, the 2nd group is 12–13 years old, and the 3rd group is 15–16 years old school children. Then each group was further divided into 2 groups, boys’ and girls’ groups. 100 of the selected children are the control group, and 80 are the group of children with hypothyroidism (subclinical). We studied children with hypothyroidism (subclinical) based on the information received by the school nurse and the polyclinic nurse. Then we measured the anthropometric indicators of children using the Bunak method. The teeth of 80 children were examined for morphological examination. For this, they were divided into 2 groups: 1st group consisted of 40 children of different ages diagnosed with hypothyroidism (subclinical) teeth removed due to caries. Group 2 consists of teeth removed due to caries from children not diagnosed with hypothyroidism (subclinical).
3. Results of the Study
In the implementation of the cynical method, we have used extensive statistical testing steps. First, we conducted a survey of selected school–aged children with their passports and outpatient information. We collected our data using the ambulatory cards kept by the school nurse (№287) and the polyclinic nurse (№025) of children in the healthy (control) group. We also studied children with hypothyroidism (subclinical) based on the information we received from the school nurse and the polyclinic nurse. Then we divided all collected data into 3 age groups (see Table 1). We divided these groups again by gender: boys and girls. First, we studied the musculoskeletal system of children in the control group. In addition, we took into account the mental and physical development of each child, whether or not they have genetic diseases. In the second group, we monitored hypothyroidism (subclinical) level, observed certain consequences, as well as the role of these consequences in the child’s life, their impact on mental and physical development. Most importantly, we took into account whether the offspring has a severe form of hypothyroidism or not. In control and experimental group of white laboratory rats, we monitored the changes on the outside of the tooth. Especially in the 60–day–old white laboratory rats in the experimental group, we can observe darkening of teeth.We used the Bunak method, not its 21 parameters, but 5 of them related to the jaw system. That is, the physiognomic height of the face, the total morphological height of the face, the morphological width of the face, the upper depth of the face, the height of the body of the lower jaw.In all of our observations, the average physiognomic height of the face of healthy school–aged boys of the 2nd grade (7–8 years old) was 18.7±0.33 cm. The average morphological height of the face of boys of the same age was 12±0.31 cm, the morphological width of the face was 22±0.68 cm, the upper depth of the face was 13±0.82 cm, and the height of the lower jaw was 3.5±0.50 cm. In girls of this age, these parameters were equal to the following parameters on average: the physiognomic height of the face was on average 17±0.97 cm, the morphological height of the face was on average 10.7±1.03 cm, the morphological width of the face was 20.2±0.84 cm, the depth of the upper jaw was 11.1±0.78 cm, and the height of the lower jaw was 3.4±0.48 cm (Diagram 1).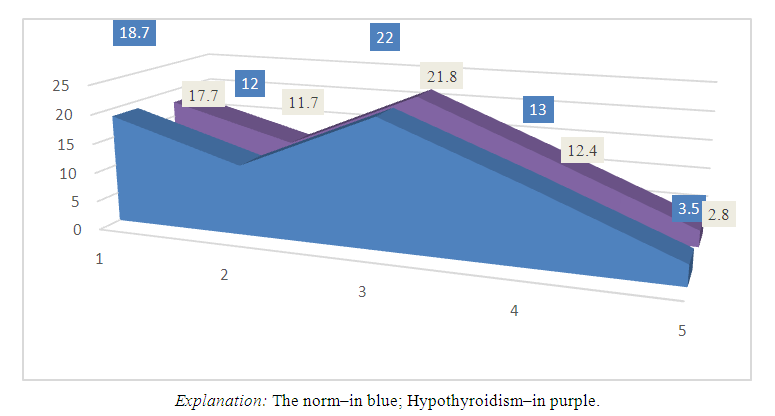 | Diagram 1. The dynamics of changes in the anthropometric parameters of the teeth and jaw system of 8–9–year–old boys |
We also studied the anthropometric indicators of the teeth and jaw system of children in the healthy (control) group of 12–13 years old. In this case, the physiognomic height of the face in the group of boys was 21.5±0.97 cm on average, and in the group of girls it was 21.8±0.85 cm. During this period, the total morphological height of the face was found to be 14.3±0.94 cm in the boys’ group, and 13.3±0.91 cm in the girls’ group. The average morphological width of the face is 24.1±0.89 cm in the group of boys and 23.4±0.70 cm in the group of girls. In the same period, the depth of the upper part of the face was 14.6±0.76 cm in the group of boys, and 13.5±0.83 cm in the group of girls. The average height of the lower jaw was 4.0±0.52 cm in the group of boys, and 4.3±0.42 cm in the group of girls.We measured the anthropometric parameters of the teeth and jaw system of 15–16–year–old healthy (control) children. The following indicators were observed, the physiognomic height of the face in the boys’ group was on average 29.5±0.73 cm, and in the girls’ group it was 23.7±0.94 cm. In the same period, the total morphological height of the face was 18.0±0.82 cm in the group of boys, and 14.9±0.84 cm in the group of girls. The average morphological width of the face is 29.4±0.79 cm in the group of boys and 25.9±0.80 cm in the group of girls. During this period, the depth of the upper part of the face is 17.2±0.83 cm in the group of boys, and 15.1±0.74 cm in the group of girls. The height of the lower jaw was found to be 5.7±0.62 cm in the boys’ group, and 5.2±0.39 cm in the girls’ group.The tooth consists of hard and soft parts. The hard part of the tooth consists of enamel, dentin and cementum. The soft part of the tooth is represented by the pulp. The thickness of the enamel crown was 3100.0±2.5 μm in the 3–day–old experimental group. It can be seen that tooth enamel consists of prisms. Prisms form tangles perpendicular to the dentin. It can be seen that enamel is covered with a thin cuticle from the outer layer. During this period, the thickness of dentin was found to be 585.1±16.54 μm, and the change of predentin thickness was observed to be 22.3±2.43 μm. Dentin consists of organic and inorganic matter. It appears that the organic part of dentin consists of collagen fibers. These collagen fibers are oriented longitudinally and radially. At the same time, the thickness of the cement is 190.5±6.7 μm. Dentin tubules consist of dentinoblast growths and tissue fluid, and the width of dentin tubule space is 835.4±5.32 μm. The thickness of the dentin tubules varies in the area of the tooth root and crown. Dentin is separated from dentinoblasts by narrow predentin. Pulp thickness changed to 3.2±0.76 μm (Figure 1).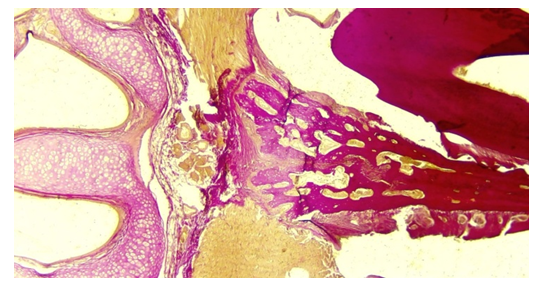 | Figure 1. Observation of the decalcification process in a rat tooth in the 60–day experimental group. Painting: van–gizon X. 10x10 |
The enamel crown thickness of 21–day–old rats in the experimental group decreased by 12% compared to 3–day, dentin thickness changed significantly by 10.6%, predentin thickness increased by 11.36%, and cementum thickness increased by 11%. It was observed that the width of the cavity of the dentin tubules was unchanged by 10.6%, and the thickness of the pulp was significantly reduced by 11.82%.30–day–old rats in the experimental group showed an 11% increase in enamel crown thickness compared to 3–day–old rats, a significant decrease in dentin thickness by 11.5%, a significant decrease in predentin thickness by 11.01%. It was found that it decreased by 10%, and pulp thickness decreased by 11.1%.The 60–day–old rats in the experimental group had a significant 10% change in enamel crown thickness compared to 3–day, a 10.25% increase in dentin thickness, a 11.26% decrease in predentin thickness, and a 10.17% increase in cementum thickness. It was observed that the width and thickness of dentin canaliculi changed significantly by 11%, and the pulp thickness significantly decreased by 10.02%.
4. Conclusions
1. It was found that the physiognomic height of the face increased by 15% in school–age 7th grade boys (aged 11–14). 2. It was noted that the morphological height of the face of boys of the same age increased by 19%, the morphological width of the face decreased by 10%, the upper depth of the face increased by 12%, and the height of the lower jaw increased by 14%. 3. These indicators were equal to the following indicators in girls of the same age: the physiognomic height of the face increased by 28%, the morphological height of the face increased by 24%, the morphological width of the face increased by 16%, the upper depth of the face increased by 22%, and the height of the lower jaw increased by 26%.
References
| [1] | Akhmedova S.M., Nortaeva N.A., Nortaev A.B. Morphological changes in the teeth of adolescent children with hypotireosis // Collection of materials of the scientific and practical conference with international participation, dedicated to the 100th anniversary of the Tashkent Medical Academy, “100 years of the Tashkent Medical Academy – the era of great achievements and discoveries”. Tashkent, 2022. – p. 199–200. |
| [2] | Mirzamukhamedov O.Kh., Akhmedova S.M. Modelirovanie toksicheskogo myocardita na fone hypothyroidism // Medical bulletin. – No. 5., Tashkent, 2019. – p. 56–59. |
| [3] | Mirsharopov U.M., Usmonov R.J., Teshaev O.R., Mirzamuhamedov O.Kh., Akhmedova S.M. et al. Morphological change of myocardium in hypothyroidism // Central Asia Journal of Medicine. – № 1., 2020. – p. 71–83. |
| [4] | Mirzamukhamedov O.Kh., Mirsharopov U.M., Sodikova Z.Sh., Akhmedova S.M., Khatamov A.I., Mirzabekova O.A. Especially the development of myocarditis in hypothyroidism in postnatal ontogenesis // Indian Journal of Forensic Medicine & Toxicology. Vol. 14, № 4, 2020. – p. 7737–7745. |
| [5] | Nortaeva N.A., Akhmedova S.M., Nortaev A.B. Morphological changes in the teeth adolescent children with hypotireosis // Problems of biology and medicine. Samarkand, 2022. – p. 270. https://doi.org/10.38096/2181–5674.2022. |
| [6] | Nortaeva N.A., Nortaev A.B. Morphological changes in teeth against the background of experimental hypothyroidism // Topical issues of modern scientific research. – Dushanbe, 2022. – p. 168. |
| [7] | Nortaeva N.A., Nortaev A.B. Morphological changes in the tooth in experimental hypothyroidism // Issues of innovative development of science, education and technology. – Andijan, 2022. – p. 273–275. |
| [8] | Nortaeva N.A., Nortaev A.B., Akhmedova S.M. To study the morphological changes in the tooth against the background of experimental hypothyroidism // Current problems of microbiology. – Tashkent, 2022. – p. 148–152. |
| [9] | Nortaeva N.A. Morphological changes in teeth as a result of malnutrition in preschool children // Proceedings of the conference dedicated to the 95th anniversary of academician, morphologist, scientist Komiljon Zufarov. – Tashkent, 2021. – p. 34–36. |
| [10] | Nortaeva N.A., Akhmedova S.M. Morphological changes in the dental in experimental hypotireosis // Collection of materials of the scientific and practical conference of young scientists with international participation, dedicated to the 100 th anniversary of the Tashkent Medical Academy, “innovative approaches in medicine”. – Tashkent, 2022. – p. 51. |
| [11] | Nortaeva N.A., Akhmedova S.M., Nortaev A.B. Anthropometric indicators of the maxillofacial system in school–aged children with hypothyroidism // Modern scientific research topical issues, achievements and innovations. Current scientific issues, current affairs, achievements and innovations. Penza, 2023. – p. 153. |
| [12] | Nortaeva N.A., Akhmedova S. M., Nortaev A.B., Rajabov B.M. Changes in the face–jaw system of experimental hypothyroidism // Texas Journal of Medical Science, 2023. – p. 61–64. https://zienjournals.com. |
| [13] | Nortaeva N., Akhmedova S., Berdiev O. Anthropometric dimensions of the maxillofacial system in children with hypothyroidism aged 8–16 year // Journal of Medicine and Innovations, 2023. – p. 230–235. www.tsdi.uz. |
| [14] | Nortaeva N.A., Akhmedova S.M., Nortaev A.B. Effects of hypothyroidism on the maxillofacial system // Uzbek journal of case reports. Том 3, 2023. – p. 126. https://doi.org/10.55620/ujcr.3.sp.2023. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML