-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 713-716
doi:10.5923/j.ajmms.20231305.35
Received: May 9, 2023; Accepted: May 24, 2023; Published: May 27, 2023

About Some Morphological Changes in the Pancreas in Experimental Hypothyroidism
Niyozov Norbek Kurbonovich1, Akhmedova Sayyora Mukhamadovna2, Nisanbaeva Aziza Urinbekovna1, Tolmasov Ruzibek Tolmasovich1
1Assistant, Tashkent Medical Academy, Uzbekistan
2Doctor of Medical Sciences, Professor, Tashkent Medical Academy, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Research has scientifically substantiated with the results of inspections conducted on 56 white laboratory rats during early postnatal ontogenesis. Complex morphological studies include general histological, histochemical methods of organometry, morphometry. For the study, we used the offspring of rats born from control and experimental white laboratory rats –mothers under conditions of hypothyroidism. The results of the study showed that the introduction of Mercazolil into the pancreatic lobules of experimental rats led to changes associated with the normalization of the structural organization of the pancreatic cranium, interlobular connective tissue with the formation of fibrous tissue components, as well as the disappearance of choroidal edema observed in the interlobular connective tissue.
Keywords: Mercazolyl, Pancreas, Morphological changes, Pieces of pancreas, Hypothyroidism
Cite this paper: Niyozov Norbek Kurbonovich, Akhmedova Sayyora Mukhamadovna, Nisanbaeva Aziza Urinbekovna, Tolmasov Ruzibek Tolmasovich, About Some Morphological Changes in the Pancreas in Experimental Hypothyroidism, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 713-716. doi: 10.5923/j.ajmms.20231305.35.
1. Introduction
- Hypothyroidism is a clinical syndrome resulting from a long–term, permanent deficiency of thyroid hormones in the body or a decrease in their biological effect at the tissue level. According to the WHO, the prevalence of open primary hypothyroidism in the population is 0.2–1%, and hidden primary hypothyroidism is 7–10% in women and 2–3% in men. 5% of cases of latent hypothyroidism become apparent within one year [1,5,6,12]. Lack of thyroid hormones leads to systemic changes in the body. Thyroid hormones produced by the thyroid gland regulate the process of metabolism, the consumption of proteins, fats, and carbohydrates, participate in the immunogenic system and thermoregulatory processes, stimulate the work of hematopoietic organs, increase oxygen consumption by cells and tissues, increase glucose consumption in gluconeogenic processes, regulates physical adaptation, adaptive reactions [2,3,4,10]. Hypothyroidism causes several disorders in all organs and systems due to various effects of thyroid hormones [7,8]. First of all, it affects the circulatory system, the digestive system (function of the liver and pancreas), the central nervous system, the organs of vision, and the reproductive system [9,11,13]. The above–mentioned points show that the problem we have chosen is dedicated to the actual problem.The purpose of the study: to determine the nature of morphological and morphometric changes in the pancreas in experimental hypothyroidism.
2. Material and Research Methods
- To achieve the goal of the study, the pancreas of 56 sexually mature white laboratory rats was studied. White laboratory rats were divided into 2 groups. Group 1 was a control group of 20 healthy rats. The rats in the experimental group were divided into 2 groups. In the experimental group, 36 female white laboratory rats were given mercazolil in the amount of 0.5 mg per 100 g of body weight for 14 days to induce experimental hypothyroidism. Later, 0.25 mg of mercazolil per 100 g of body weight was given to rats for 1 month. Mercazolil was administered at 0.25 mg per 100 g of body weight during the nursing period after the rats became pregnant and after the birth of their offspring. Blood was taken from the tail vein of mother and baby rats, and the amount of thyroid hormones was studied. Baby rats were killed by decapitation on the 3rd, 7th, 14th, 21st, and 30th days after birth. For histological examinations, tissues were taken from the head, body, and tail of the pancreas. Pancreas tissue was fixed in 10% formalin solution, dehydrated in alcohol, and paraffin blocks were prepared. 8–12 μm histological preparations were prepared from the prepared paraffin blocks and stained by the hematoxylin–eosin method. Experiments and decapitation of animals were performed by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1985). Histological sections prepared on a rotor microtome with a thickness of 8–10 microns were stained with hematoxylin–eosin in a standard way [Volkova O.V.V., Yeletsky Yu.K., 1982].
3. Results
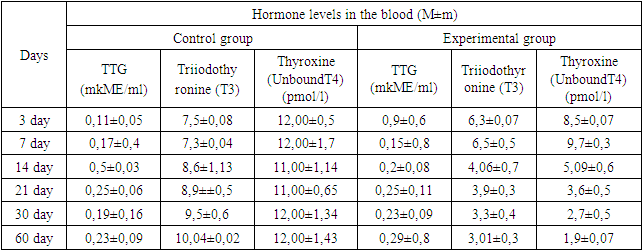
- To justify the development of experimental hypothyroidism in rats, the amount of triiodothyronine (T3), unbound thyroxine (T4), and thyrotropin hormone (TTG) in the blood of rats were determined on different days of the experiment. The obtained data showed that on the 7th day of the experiment, the T3 and T4 hormones of the experimentally induced hypothyroidism and control group rats did not differ from each other. On the 14th day of the experiment, there was a decrease in T4 and a smaller decrease in T3. On the 21st day of the experiment, it was found that the indicator of T4 hormone decreased by 2 times, and T3 decreased by 1 time. Thyroid hormones in the blood of 30–day–old rats changed by 4 times according to the T4 index, and T3 decreased by one and a half times. Thus, the analysis of the hormone indicator showed a reliable decrease in the indicator of the hormone thyroxine (T4) in the blood of rats in the state of experimental hypothyroidism. The reduction of the T4 hormone was reflected from the 14th day, and in the last 30 days of the experiment, the reliability decreased to 4 times. The amount of thyroid hormones in the blood is controlled by thyrotropin. A decrease in the amount of T3 and T4 hormones in the blood led to an increase in the TTG hormone. On days 3 and 7, the amount of TTG was not significantly different from the control group. By the 14th day of the experiment, a gradual increase in TTG was noted, and by the 21st day, it was twice as high as in the control group (Table 1).
|
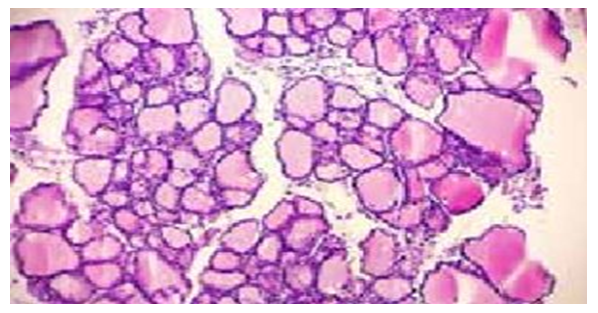 | Figure 1. Different types of follicles. Thyroid gland of a control rat. Staining: hematoxylin–eosin. X:10x40 |
 | Figure 2. View of the rat pancreas |
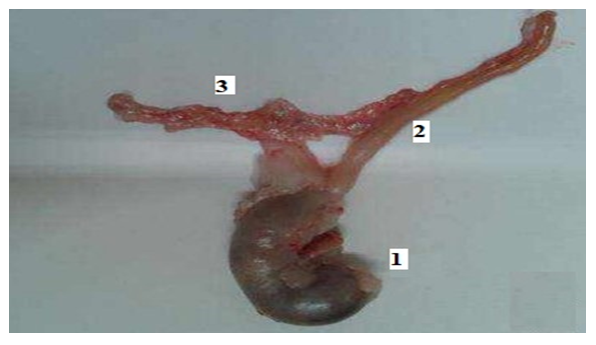 | Figure 3. 1–stomach, 2–duodenum, 3–location of pancreas in rats |
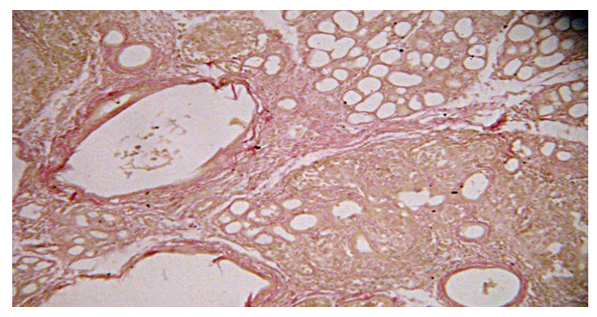 | Figure 4. Rat pancreas on the 14th day of the experiment. Enlargement of the pancreatic ducts. Color: by Van Gieson |
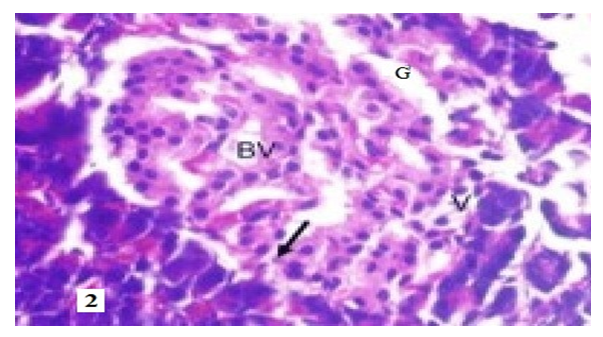 | Figure 5. BV–dilated capillary blood vessels, V–cytoplasmic vacuolization, G–eosinophilic stained cytoplasm pyknotic nucleus, arrow karyolytic nucleus. Staining: hematoxylin–eosin. X: 10x40 |
4. Conclusions
- The results of the study showed that the introduction of Mercazolil into the pancreatic lobules of experimental rats led to changes associated with the normalization of the structural organization of the pancreatic cranium, interlobular connective tissue with the formation of fibrous tissue components, as well as the disappearance of choroidal edema observed in the interlobular connective tissue. In addition, the intensity of symptoms of the destruction of the terminal secretory section of the lobules decreased and at the same time, the number and height of the pancreas in the lobules increased. This may be due to the intensification of the process of division of the pancreas and the activation of the secretory process. In the endocrine part of the gland lobules, a thickening of the location of insulocytes in the islets and a decrease in areas filled with a loose connective tissue layer were observed, in addition, the size of the islets increased and became larger than in the control animals. This may indicate a general increase in the number of endocrine cells in the gland, and hence an increase in hormone production.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML