-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 708-712
doi:10.5923/j.ajmms.20231305.34
Received: May 7, 2023; Accepted: May 22, 2023; Published: May 27, 2023

Study of Anti-Inflammatory Activity "Dorusim"
Khakimov Z. Z. 1, Rakhmanov A. Kh. 1, Babazhanov A. U. 2, Tursunova L. I. 3, Khadzhieva U. A. 3
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Urgench Branch of the Tashkent Medical Academy, Tashkent, Uzbekistan
3Uzbek Research Chemical and Pharmaceutical Institute named after. A. Sultanov, Tashkent, Uzbekistan
Correspondence to: Rakhmanov A. Kh. , Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The results of experimental studies conducted on adult white male rats showed that the phytocomposition Dorusim has a pronounced anti-exudative activity in aseptic arthritis induced by various phlogogens. An effective anti-inflammatory dose of Dorusim in enteral administration is 50 mg/kg. A possible mechanism of the anti-inflammatory activity of Dorusim is its suppression of the release of histamine from mast cells and the synthesis of prostaglandins.
Keywords: Aseptic arthritis, Phytocomposition, Medicinal plants, Antiinflammatory agents
Cite this paper: Khakimov Z. Z. , Rakhmanov A. Kh. , Babazhanov A. U. , Tursunova L. I. , Khadzhieva U. A. , Study of Anti-Inflammatory Activity "Dorusim", American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 708-712. doi: 10.5923/j.ajmms.20231305.34.
Article Outline
1. Introduction
- Recently, the problem of drug safety has become increasingly relevant worldwide due to the fact that mortality caused by adverse reactions ranks 5th globally, following diseases of the cardiovascular system, lungs, oncological pathologies, and injuries [1]. Modern medicine has a wide arsenal of anti-inflammatory drugs, but their regular use leads to the development of a number of side effects such as gastro-, nephro-, cardio-, hemato-, hepatotoxicity, which is associated with their ability to penetrate through histohematic barriers and the manifestation of systemic action [2,3,4,5,6,7,8]. Therefore, the search for new compounds with anti-inflammatory action is an urgent task of pharmacology. In recent years, interest in medicinal plants has increased significantly due to the fact that the use of synthetic drugs is accompanied by various side effects, and on the other hand, a high content of biologically active substances in the complex. Literature data indicate that medicinal plants: Herba alhagi, Folium Uvae ursi, Fructus Rosae, Glycyrrhiza glabra and Flores chamomillae have antioxidant and membrane stabilizing effects [9,10,11,12], which allows us to assume that this phytocomplex has an anti-inflammatory effect. The literature data show that this phytocomposition has not been specifically studied as an anti-inflammatory agent.The purpose of this work was to study the anti-inflammatory activity of Dorusim.
2. Material and Methods
2.1. Experiments
- Experimental studies were carried out on adult white male rats weighing 145-160 g, obtained from the vivarium of the Department of Sanitary and Epidemiological Surveillance of the Main Medical Department under the Administration of the President of the Republic of Uzbekistan. Prior to the start of the experiment, all animals were examined, weighed after a two-week quarantine. Age, sex, physical activity and skin condition of animals were taken into account. Each experimental and control group consisted of six individuals. During experimental studies, laboratory animals were kept in a vivarium in plastic cages, bedding of sawdust at a temperature of 20-24°C, in a well-ventilated room and day/night light regimen. Humidity was at least 50%. Feeding of animal was calculated according to their age. For the study, we selected extracts of the following medicinal plants Herba alhagi, Folium Uvae ursi, Fructus Rosae, Glycyrrhiza glabra and Flores chamomillae, and it was conditionally named "Dorusim". Diclofenac sodium (Belmedpreparaty) and Canephron (Bionorica SE., Germany), which is a mixture of dry extracts of medicinal plants, were used as a reference drugs. The classical models of experimental aseptic arthritis induced by a formalin (2%), carrageenan (1%), dextran (6%) and histamine (0,1%) were used to study the anti-exudative activity of the above compositions of dry extracts of medicinal plants [13,14,15,16]. The phlogogen solutions were injected (0.1 ml per animal) subplantarly (under the plantar aponeurosis) into the hind right paw of rats. [13]. The volume of paws of rats before the injection of the phlogogen was considered initial and was taken as 100%. One day and 1 hour before the induction of aseptic arthritis, rats of the control group were administered intragastrically an equivolume amount of water as well as animals of the experimental groups were administered the Dorusim in various doses of 25, 50 and 100 mg/kg, canefron - 100 mg/kg and sodium diclofenac - 10 mg/kg. The volume of the paws of the animals was measured by the oncometric method using a plethysmometer (Ugo Basile Srl, Italy) before and after the injection of phlogogens. The anti-inflammatory activity of the studied compounds was judged by the difference in paw volume before the start of the experiments and at the moment of maximum development of edema. The value of anti-inflammatory activity (AIA) of the preparations was calculated according to the formula (5):AIA = Vcon – Vexp / Vcon х 100 = %where; V con - average increase in paw volume in control group, cm3, Vexp –average increase in paw volume in experimental group, cm3.
2.2. Statistical Analysis
- The data obtained were processed by the method of variation statistics using the paired Student's test and one-way analysis of variance using the standard software package BIOSTAT 2009 with an assessment of the significance of indicators (Mean±Std error). Differences in the compared groups were considered significant at a significance level of 95% p <0.05.
3. Results and Discussion
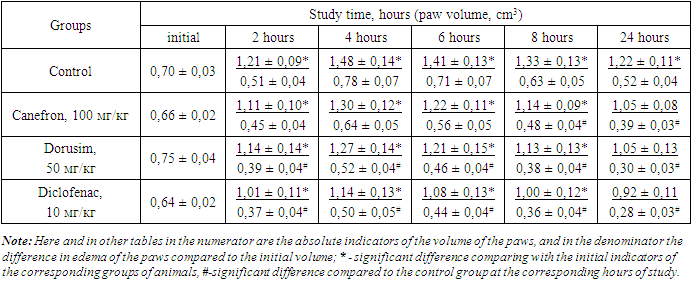
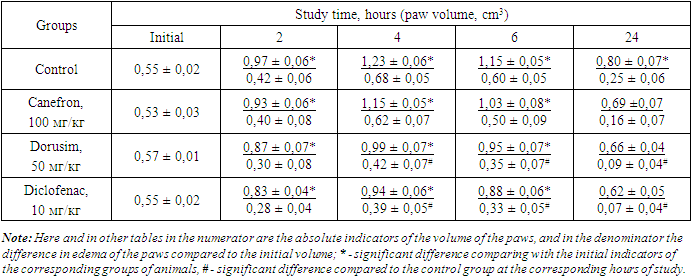
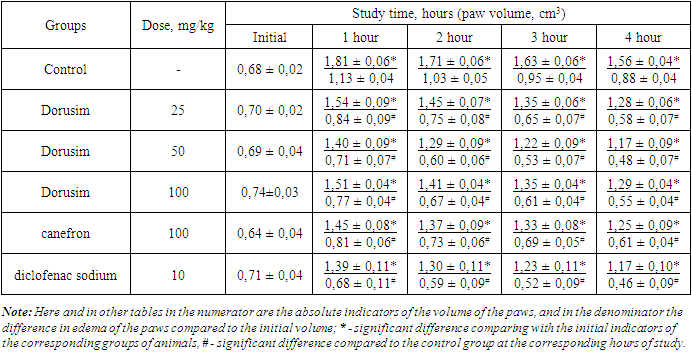
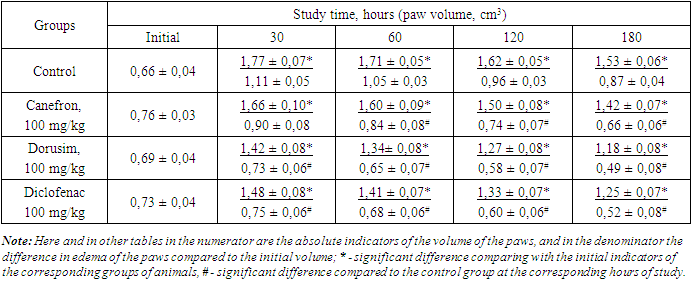
- According to the requirements of preclinical studies for new compounds with anti-exudative action, it is necessary to conduct tests on various models of aseptic arthritis induced by various phlogogens [17].The phlogogenic effect of formalin, as is known, is due to its interaction with the amino groups of proteins and the release of biogenic amines and free amino acids, followed by a disturbance of isoionia and isotonia at the injection site [13,18,19] Analysis of the results of the studies conducted on this model showed that 2, 4 and 6 hours after the phlogogen injection a distinct aseptic arthritis developed under the influence of formalin, which manifested in an increase in the volume of the paws of rats by 72.8; 114.4 and 101.4%, respectively. At the same time, the noted effect with slight fluctuations (increase by 90.0 and 74.3%) persists after 1 and 2 days from the start of the experiment. As can be seen from the data in Table 1, the preliminary administration of diclofenac sodium inhibits the development of paw edema 2, 4, 6, 24 and 48 hours after injection of formalin compared with the control group by 20.4; 29.1; 32.2; 37.6 and 41.2%, respectively.
|
|
|
|
4. Conclusions
- 1. The phytocomposition Dorusim has a pronounced anti-exudative activity in aseptic arthritis induced by various phlogogens.2. The effective anti-inflammatory dose of Dorusim in enteral administration is 50 mg/kg.3. In terms of its pharmacological activity, Dorusim is not inferior to sodium diclofenac and is noticeably superior to the phytopreparation Canefron.4. A possible mechanism of the anti-inflammatory activity of Dorusim is its suppression of the release of histamine from mast cells and the synthesis of prostaglandins.
ACKNOWLEDGEMENTS
- The work was supported by grant PZ-202102233.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML