-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 702-707
doi:10.5923/j.ajmms.20231305.33
Received: Apr. 28, 2023; Accepted: May 21, 2023; Published: May 27, 2023

Algorithm for Identifying Risk Factors for Ovarian Hyperstimulation Syndrome
Kurbaniyazova Madina Zafarjanovna1, Azhetova Zhanerke Rakhimbaevna2, Saparbayeva Nasiba Rakhimbayevna1, Askarova Zebo Zafarjonovna3
1Assistant of the Department of Obstetrics and Gynecology, Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan
2Candidate of Medical Sciences, Associate Professor of the Department of Obstetrics and Gynecology No. 1 of NJSC "Astana Medical University" Republic of Kazakhstan, Astana, Kazakhstan
3Assiatant of the Department of Obstetrics and Gynecology №1, Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This problem of infertility is of great medical and socio-economic importance. First, OHSS is a disabling and potentially fatal iatrogenic complication. Patients with OHSS stay in hospital for a long time in wards and spend a lot of money on medicines, a woman is away from her family, work, and even causes disability. Predicting the risk of developing ovarian hyperstimulation syndrome in women is one of the most important areas in the development of modern medicine. The modern development of medicine should consider OHSS as a preventable complication of ovulation induction, so the main prevention of this syndrome is the assessment of risk factors at the first stage, that is, before stimulation, and the correct choice of drugs for ovulation induction. At the second stage, i.e. stage of direct stimulation, predictors of OHSS may be determined, ovulation triggers may be selected and medications may be used, or other tactics may be used, as indicated below.
Keywords: Algorithm, Ovarian hyperstimulation syndrome, Predictors, VEGF, ORPI
Cite this paper: Kurbaniyazova Madina Zafarjanovna, Azhetova Zhanerke Rakhimbaevna, Saparbayeva Nasiba Rakhimbayevna, Askarova Zebo Zafarjonovna, Algorithm for Identifying Risk Factors for Ovarian Hyperstimulation Syndrome, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 702-707. doi: 10.5923/j.ajmms.20231305.33.
Article Outline
- Ovarian hyperstimulation syndrome (OHSS) is the most dangerous complication of ovulation induction associated with severe pathological reactions, and multiple organ failure even leads to fatal consequences. According to the Practical Committee of the American Society for Reproductive Medicine (2016), the incidence of all types of OHSS is 33%, of which 1–5% are moderate and severe [3].Infertile women with normal body weight and PCOS often respond to ovulation stimulation with good and sometimes excessive follicle growth. The response of the ovaries to drugs in many cases depends on the dose of gonadotropins.Early identification of predictors of an acute ovarian response helps to select the correct dose of drugs used for stimulation, which in turn leads to a reduction in complications such as missed induction cycles due to overreaction of the ovaries, multiple pregnancies and ovarian hyperstimulation. Strong response to OHSS Ovulation induction is accompanied by ovarian secretion of VEGF, a vasoactive angiogenic compound that increases capillary permeability and causes fluid to accumulate in the extravascular space. The basic classification of OHSS was developed by Golan and modified in 2021. The classification distinguishes 3 categories of severity and 5 degrees of severity [12].
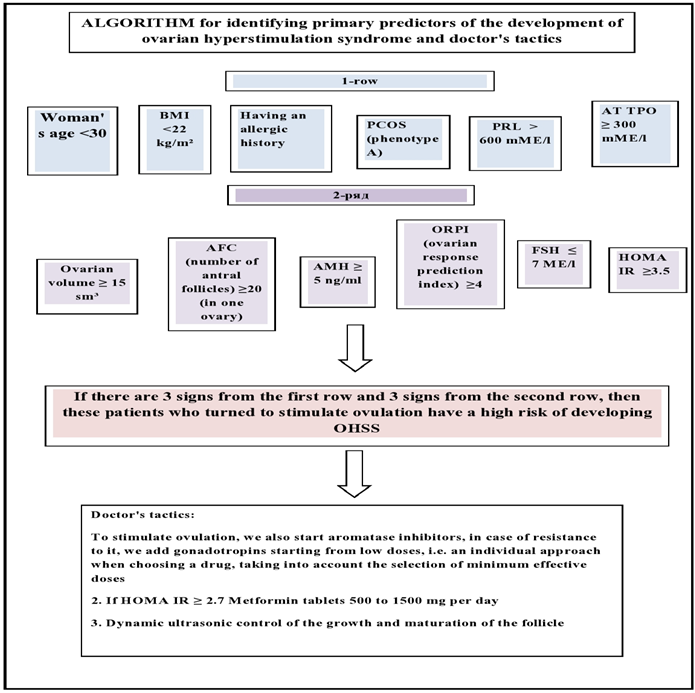
1. Modified Golan Classification of OHSS
- I Mild OHSS: characterized by bilateral ovarian enlargement (multifollicular enlargement).- 1st degree: flatulence and abdominal discomfort;- 2nd degree: in addition to the above - nausea, vomiting and / or diarrhea + ovarian enlargement from 5 to 12 cm.II Medium OHSS: abdominal enlargement due to ascites;- 3rd degree: mild OHSS signs + ultrasound signs of ascites.III Severe OHSS: characterized by hypovolemia, hemoconcentration, thrombosis, oliguria, fluid accumulation in the pleura and pericardium.- 4 degree: moderate OHSS + clinical ascites and/or hydrothorax and shortness of breath.- 5 degrees: 4 degrees + changes in blood volume, hemoconcentration, clotting disorders and decreased renal perfusion.The incidence of OHSS in the population: the frequency of mild OHSS is from 8 to 23%, the frequency of moderate form is from 1 to 7%, the frequency of severe forms of ovarian hyperstimulation syndrome is from 1 to 10%.The risk of ovarian hyperstimulation syndrome can be assessed both before ovulation stimulation and during a stimulated cycle.Prior to the start of treatment for anovulation, that is, the primary predictors of the development of OHSS are:
2. Primary Predictors of the Development of OHSS (Table 1)
- Patient's ageThis determines the greatest risk of developing OHSS. So, at the age of 18-25 years, young women who have not undergone surgery on the pelvic organs and do not have endometriosis are prone to developing OHSS, since the gonads at this age are most sensitive to gonadotropins. On the surface of the ovaries there are receptors for gonadotropic hormones, and in a young woman who has just begun sexual activity, episodes of inflammatory diseases are observed less frequently than in older women, since inflammatory processes become chronic with age and this leads to deterioration of receptors in the ovaries, in therefore, a large number of follicles sensitive to gonadotropins appear at a young age [6,10].Body mass indexThe study showed that the induction of ovulation in underweight women leads to the development of many follicles and, thus, to an increase in the concentration of estradiol in the blood. Studies show that the development of OHSS occurs more often with a BMI ≥ 22 kg/m2 [10].Polycystic ovary syndrome (PCOS)The incidence of ovarian hyperstimulation syndrome has been shown to be highest in patients with PCOS. Polycystic ovary syndrome is a proven risk factor for the development of OHSS. Increases ovarian follicular activity along with insulin resistance and hyperinsulinemia, which leads to an increase in vascular endothelial growth factor - VEGF. A 2013 study showed that insulin is a factor that increases VEGF production [13,18].HOMA-IRCalculation of the index of insulin resistance according to the following formula: it is determined based on the concentration of glucose and insulin in the serum of peripheral blood on an empty stomach before the start of ovarian stimulation;HOMA-IR = fasting insulin (mU/ml) × FG (mmol/l): 22.5;where FI is insulinemia on an empty stomach; FG, fasting glycemia; 22.5 is a constant coefficient.Women with PCOS have a significantly higher rate of insulin resistance, suggesting they should consult an endocrinologist.Our study found a significant moderate correlation between HOMA-IR score and development of OHSS and allowed us to define a cut-off level of HOMA-IR of 2.7 as a valuable predictive marker for the development of OHSS.Insulin resistance is one of the first signs of an increased risk of developing OHSS upon ovulation induction, therefore, patients with threshold levels of HOMA-IR should be carefully prepared, including mandatory consultation with an endocrinologist and special training (oral hypoglycemic drugs - taking metformin is considered) [5,9].Functional hyperprolactinemiaHyperprolactinemia plays a special role in the pathogenesis of OHSS. Hyperprolactinemia is a polyetiological condition, manifested by an increase in the concentration of prolactin in the blood above 600 mU / l. There are several forms of hyperprolactinemia syndrome, depending on the cause. Primary hyperprolactinemia can result from disorders in the physiology or anatomy of the hypothalamic-pituitary system, for example: benign and malignant neoplasms, inflammatory processes, exposure to chemical or radiation factors, and traumatic injuries. Secondary pathological hyperprolactinemia is always the result of another disease, such as PCOS. The pathogenesis of the development of ovarian hyperstimulation syndrome against the background of hyperprolactinemia is associated with the influence of dopamine. Dopamine is the main inhibitor of prolactin. Violation of the synthesis and secretion of dopamine leads to hyperprolactinemia. At the same time, dopamine is an inhibitor of VEGF production. Therefore, impaired dopamine synthesis in hyperprolactinemia is a factor that increases the risk of developing OHSS. The drug cabergoline is a dopamine agonist used to treat hyperprolactinemia. The drug cabergoline reduces vascular permeability and is prescribed within 4 days from the day of taking the ovulation trigger, with meals before bedtime [9,12].AT TPO - antibodies to thyroperoxidaseSince thyroid pathology is very common in European and Asian populations, the role of thyroid pathology in the pathogenesis of OHSS is very important. On the one hand, there is very little evidence in the scientific literature about an increased risk of developing OHSS in women with impaired thyroid hormone levels. It is caused by thyroid-stimulating hormone-mediated stimulation of follicle-stimulating hormone receptors. Anti-thyroid peroxidase antibodies (AT TPO) were higher in women with PCOS and insulin resistance. Also Zhukovskaya S.V. 2019, as a result of studies, the concentration of TPO antibodies was ten times higher in women who underwent ovulation stimulation, which was associated with the development of OHSS.Ovarian volumeIn patients scheduled for emergency cardiac surgery, baseline ovarian volume was measured using 2D-3D ultrasound. There was a strong correlation between the initial size of the ovaries and the subsequent development of OHSS (p = 0.03); Baseline volume was significantly higher in patients with OHSS (8.9 cm3 versus 11.3 cm3). The authors did not elaborate on their data, but concluded that ovarian sizing helps identify patients at risk of OHSS and prevent it by predicting hormone doses early [17–18]. The limiting volume is estimated at about 10 cm³. OHSS is 10% in patients with a volume of less than 10 cm³, 23.5% in patients with a volume of more than 10 cm³. The study did not show the size of the ovary, in which OHSS is absent at all or occurs in a very small number of patients [4].Number of antral folliclesThe number of antral follicles (AFC), determined by ultrasound on days 2-3 of the menstrual cycle, accurately reflects the current state of the ovarian reserve. Antral follicles are small follicles (2-8 mm in diameter) that we can see, measure and count on ultrasound. If AFC is up to 5, then the ovarian response is extremely poor, if AFC is 8-12, then this indicates a moderate response, if AFC is 13-20, then this is a good response (moderate risk of developing OHSS). more than 20 indicates an overreaction of the ovaries, which means a high risk of developing OHSS.Anti-Müllerian Hormone (AMH)The role of the AMH hormone in predicting the risk of developing OHSS has been confirmed by many research authors. According to various sources, AMH ≥ 3.5 nmol/l is a threshold value, when it is exceeded, the risk of developing OHSS increases. It should also be noted that AMH levels are elevated in women with PCOS, which is associated with a greater development of antral follicles compared to healthy women. This fact once again confirms that PCOS is at risk for the development of OHSS [15]. The amount of AMH is low at its level from 1 to 3.5 nmol / l, normal - from 3.5 to 13 nmol / l and high at its level of 13 nmol / l or more (this is a high risk of developing OHSS).ORPI (ovarian response prediction index)ORPI = (AMH x AFC)/ageThis indicator shows the response of the ovaries to ovulation stimulation, and in women with PCOS, if this indicator is 4 or more, this indicates a very high probability of developing OHSS [6,13].Allergological historyHypersensitivity of the immune system, OHSS may also indicate the risk of developing allergies. Studies have shown that women with OHSS complications were more likely to suffer from allergies than the control group (56% versus 21%) [6]. According to the authors, there may be a link between allergy and OHSS, since the pathophysiological changes in the ovaries in OHSS are similar to an excessive inflammatory response, but the effect of allergy on the development of OHSS requires additional evidence.
|
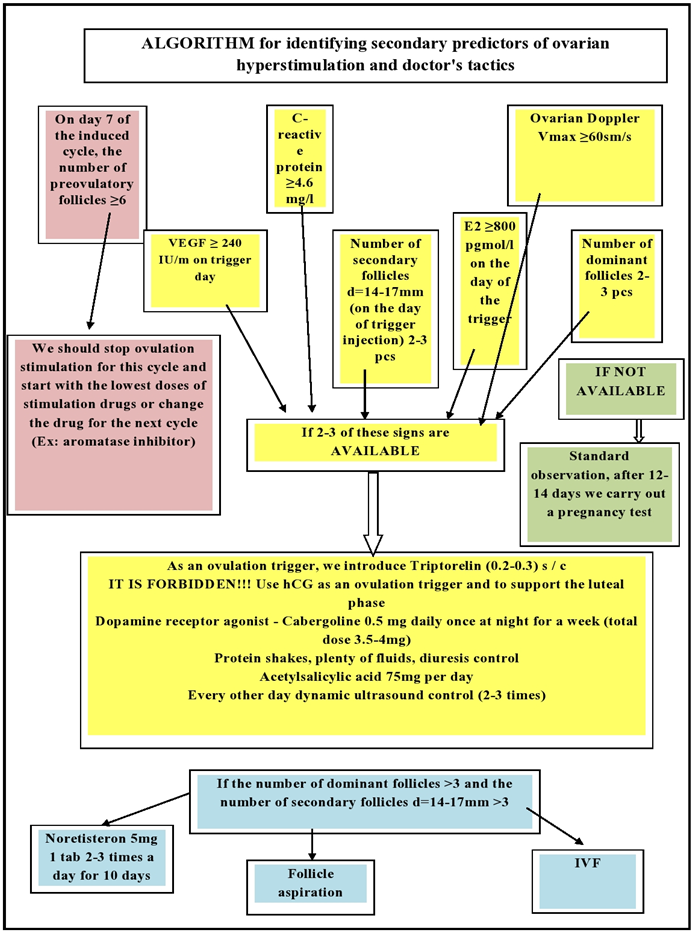
3. Secondary Predictors of the Development of OHSS
- Secondary risk factors for OHSS are those that become apparent only after ovulation induction has begun (Table 2).Estradiol (E2)An increase in serum E2 (estradiol) levels during ovarian stimulation or on the day of hCG (human chorionic gonadotropin) administration is an important risk factor for developing OHSS [7,8]. A high level of E2 is often associated with a large group of follicles that are able to respond to the introduction of hCG. The cut-off level for predicting the risk of the syndrome remains unclear. Ash et al showed 38% of cases of severe OHSS at levels >6000 pg/mL in the IVF cycle. The content of E2 was measured in the middle of the follicular phase (day 9) against the background of GnRH (long-term protocol with a gonadotropin-releasing hormone (GnRH) agonist and the start of human menopausal gonadotropin (hMG) on the current day), if its level was > 4000 pg /ml, OHSS was observed on the day of hCG administration. On day 9 of the stimulation cycle, 55.8% of patients with E2 > 800 pg/mL met the criteria for ovarian hyperreactivity, with none of them developing OHSS at <300 pg/mL. According to the data obtained, the authors recommend to reduce the dose of hMG on the 9th day for the prevention of OHSS [17].It has also been shown that an important risk factor for OHSS is the rate of increase in E2 levels during stimulation with gonadotropins. For example, Enskog et al. [2,6] consider an increase in the E2 level of more than 75% compared to the previous level as an indicator of high risk. Delvin and al [12] used a mathematical model with the analysis of the slope of the E2 level curve to assess the risk of developing the syndrome and obtained positive predictive results in 76.1% of cases with 18.1% of false negative results. Thus, there is a role for E2 in the pathogenesis of OHSS, and it needs to be identified before a trigger is applied.Number of growing and dominant folliclesIt has been established that in the ovaries of patients with OHSS on the day of the introduction of an ovulation trigger, there were significantly more follicles than in women without this syndrome [5]. The number of follicles that require stopping the stimulation cycle varies from study to study. Jayaprokasan et al [1,4] showed that the risk of hospitalization for OHSS is 15% in patients with dominant follicle count ≥ 20, but less than 0.1% when follicle count is 20 in IVF protocols. Verwod et al. [2] consider 29 follicles to be the threshold number for cryopreservation of all embryos (treatment should be stopped). Some groups of scientists point to the need to control the number of follicles with a diameter of > 15 mm, while others associate the risk of developing OHSS with a diameter of ≥ 12 mm [6,13]. When predicting OHSS in induced cycles, both the total number of follicles and the number of medium-sized follicles should be taken into account. When ovulation was stimulated with clomiphene citrate or hMG, Tal et al. found that OHSS did not develop when only dominant follicles (≥ 18 mm) or one secondary follicle (14-16 mm) were present on the day of hCG administration [11]. However, in the presence of three or more secondary follicles, the frequency of OHSS increased significantly. Thus, there is a correlation between the number of follicles of medium diameter (12-14 mm) and OHSS of varying severity [9].The role of the use of human chorionic gonadotropin in the development of OHSSThe use of hCG as a trigger in the presence of secondary follicles with a diameter of 14 mm or more increases the risk of developing OHSS. hCG has a longer half-life, has a higher affinity for receptors, and lasts longer than endogenous LH [12]. Therefore, its administration leads to massive luteinization, the development of multiple corpus luteum, and the concentration of estrediol exceeds the physiological level. Similarly, the use of hCG for luteal phase support in ovulation induction protocols increases the risk of developing OHSS. Since hCG contributes to the development of OHSS, the risk of developing this syndrome increases with the onset of pregnancy, especially in multiple pregnancies [5,14].The use of a GnRH agonist as an ovulation trigger (instead of hCG) in an ovarian stimulation protocol reduces the risk of developing OHSS. The use of a GnRH agonist as a trigger stimulates the release of endogenous LH, which leads to egg maturation and ovulation.The reason why a GnRH agonist is used as a trigger instead of hCG in women at risk of OHSS is that it shortens the half-life of LH and has a direct inhibitory effect on corpus luteum metabolism.Vessels endothelial growth factor - VEGF The risk of OHSS is associated with an increase in the level of vessels endothelial growth factor (VEGF) in the blood serum and the clinical course of the syndrome [4,6]. In addition, elevated VEGF levels increase the permeability of follicular and ascitic fluid in women with OHSS [4]. Unlike natural cycles, cycles complicated by OHSS are associated with increased production of VEGF by the ovaries, resulting in increased levels of free VEGF in the early luteal phase in women at risk for OHSS.High concentration of FSHAgrawal et al [6] found that FSH, like hCG, increased VEGF production. This suggests a role for FSH levels in the pathogenesis of OHSS and is consistent with the results of a study showing a dramatic increase in FSH levels before hCG in women with significantly enlarged ovaries after hCG.C-reactive proteinC-reactive protein (CRP) is produced in the liver and is a biological marker of systemic inflammation. Its level is not subject to daily fluctuations, remains stable for a long time and increases after taking estrogens [4,9]. Recent data on an increase in the level of C-reactive protein, reflecting a systemic inflammatory response to estrogens, suggest the role of systemic inflammation in the pathogenesis of OHSS and consider a high level of C-reactive protein as a risk factor for the development of this syndrome.
|
4. Conclusions
- The conducted studies make it possible to identify predictors of ovarian hyperstimulation syndrome in the primary, that is, before ovulation stimulation, and in the secondary, that is, in the stimulated ovulation cycle. When assessing predictors, ovulation stimulation was performed with an aromatase inhibitor and/or a low dose of gonadotropins in women belonging to the high-risk group, and women with insulin resistance were prescribed Metformin at a dose of 500-1000 mg per day, a comparative analysis of the effectiveness of standard therapy. As a trigger for women at high risk for secondary symptoms, a GnRH agonist was used instead of traditional hCG, a dopamine receptor antagonist was prescribed, and thus the effectiveness of prevention of OHSS was studied.The presented results made it possible to prevent complications by timely detection of its predictors in women with anovulatory infertility, who belong to the high-risk group of OHSS and need ovarian stimulation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML