Ganieva Shakhzoda Shavkatovna, Haydarova Sarvinoz Akramovna, Yaxyoeva Feruza Obidovna
Bukhara State Medical Institute, Uzbekistan
Correspondence to: Ganieva Shakhzoda Shavkatovna, Bukhara State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Objective: To study the immuno-inflammatory markers of coronary heart disease, arterial hypertension and their combined course. Materials and methods: The research work included 116 patients with cardiovascular disease of middle and old age. The average age of patients is 62.4±1.27. All patients were examined at the Bukhara branch of the Republican scientific and practical Center for emergency medical care. Results: During the analysis, it was found that the level of SAP directly depends on the concentration of fibrinogen -r= 0.30 and oppositely depends on the concentration of PCT - r =-0.3 and IL-6-r=-0.26. At the same time, a noticeable positive association of heart rate with creatinine in the blood -r=0.35 was also revealed. The established connections show the contribution of inflammation syndrome (immune) in the progression of hypertension, or rather, indicators of the progression of hypertension in coronary heart disease are creatinine, fibrinogen, PCT and IL-6. Conclusion: Due to the high and noticeable correlation with the studied immuno-biochemical parameters of blood and functional parameters of the heart, IL-6 is a more informative indicator of the progression of coronary heart disease with the risk of complications and multiple organ failure.
Keywords:
Cardiovascular diseases, Ischemic heart disease, Arterial hypertension, Immunity
Cite this paper: Ganieva Shakhzoda Shavkatovna, Haydarova Sarvinoz Akramovna, Yaxyoeva Feruza Obidovna, Comprehensive Assessment of Immuno-Inflammatory Markers of Cardiovascular Diseases, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 581-585. doi: 10.5923/j.ajmms.20231305.07.
1. Introduction
Cardiovascular diseases (CVD) occupy one of the main places in the structure of the main etiopathogenetic factors of deterioration of patients' health, reduction of their quality of life and overall mortality of the population. This trend in the epidemiological situation is mainly determined by the increase in the incidence and complications of CVD, which are diagnosed in almost 35-50% of the adult able-bodied population. Timely detection of the disease and its causes is very important [9]. The consolidation of immuno-inflammatory, genetic and other theories of atherogenesis can be based on deterministic overexpression of cytokine genes affecting production levels, biochemical activity of immune system mediators, expression of adhesion molecules, transformation of monocytes/macrophages and smooth muscle cells into foam cells with subsequent deposition of cholesterol crystals in the intima of blood vessels [12]. Objective: To study the immuno-inflammatory markers of coronary heart disease, arterial hypertension and their combined course.
2. Materials and Methods of Research
The study included 116 middle-aged and elderly patients with an average age of 62.4±1.27 years. All patients were examined for cytokine status: IL-17A, TNF-α, C3 complement component, VEGF and a marker of the acute phase of inflammation – procalcitonin (PCT), the lipid spectrum of blood was studied, an echocardiogram (ECHOCG) and a biochemical blood test were performed.The inclusion criteria were patients aged 45 to 74 years with a diagnosis of arterial hypertension (AH), coronary heart disease (CHD), confirmed by clinical and laboratory-instrumental methods, hospitalized in a hospital. The patients of the study groups were comparable in age, gender, and the presence of CVD risk factors. AH verification was carried out according to the requirements of the World Health Organization (WHO), classified according to the International Classification of Diseases (ICD-10). At the same time, the ACC/AHA Hypertension Guidelines (2017) classification was adhered to.The exclusion criteria from the study were patients with acute myocardial infarction, acute coronary syndrome, acute infectious diseases, myocarditis and cardiomyopathies, chronic renal and hepatic insufficiency, pulmonary hypertension, congenital and acquired heart defects, systemic diseases, oncological and hematological diseases. The research was carried out in accordance with the Helsinki Declaration. Statistical processing of the results was carried out using Excel programs from the Microsoft Office XP application package (Microsoft, USA), correlation analysis was carried out using the Pearson method and evaluated on the Cheddock scale.
3. Results and Discussion
To develop specific indicators of the progression of AH in CHD, a correlation analysis of the relationship of the studied blood parameters with the parameters of ECHOCG in patients with CHD selected for examination was carried out.The dependence of the aortic diameter on the studied immunological parameters of the blood was established: a weak positive relationship with C3- r =0.20, with IL-17A- r=0.21, with PCT- r= 0.25, a negative relationship between the aortic diameter and TNF-a-r=-0.21, VEGF-r=-0.21.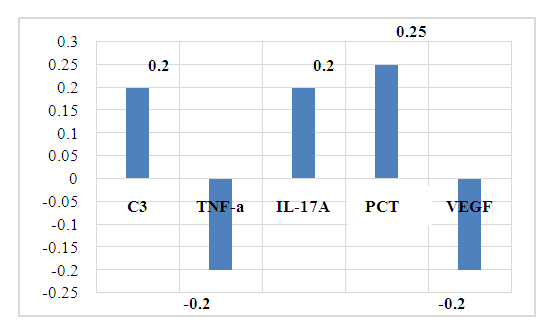 | Figure 1. Relationship of aortic diameter with immuno-inflammatory markers in CHD |
The established relationships show a directly proportional positive dependence of the aortic diameter on the level of inflammatory markers: complement C3, IL-17A and PCT (r=0.2-0.25).Currently, complement C3 is known to be a protein of the acute phase of inflammation, is involved in ensuring the body's resistance to bacterial infections and is necessary for the implementation of cytolysis and anaphylaxis.IL-17 belongs to proinflammatory cytokines and is involved in many stages of the immune response. It stimulates the production of chemokines and, as a result, stimulates the migration of neutrophils to the site of inflammation. IL-17 performs an important physiological function, participating in the protection of the body from bacterial and fungal infections [2,13].The PCT is a precursor of calcitonin and is considered a marker of sepsis. In severe bacterial infections and sepsis, the massive formation of endotoxins, an increase in the levels of proinflammatory cytokines IL-6 and TNF-α leads to an increase in the synthesis of PCT primarily in leukocytes, monocytes, as well as in neuroendocrine cells of the lungs, intestines and liver. The diagnostic value is not only the presence of an increase, but also the degree of increase, and the dynamics of the PCT level. Therefore, it is important to take into account the increase in PCT, which does not occur with fungal and viral infections, allergic and autoimmune diseases, which allows for differential diagnosis of these conditions. At the same time, constantly increasing indicators of PCT indicate a poor prognosis of the disease. An increase in the level of PCT in the blood above 1.8 ng/ml indicates the development of infectious complications (sensitivity — 80-95%, specificity — 88-93%). In patients of this group, the average concentration of PCT was 0.2 ± 0.01 ng/ml, which indicates the absence or low risk of infectious complications. The established weak positive association of PCT with the diameter of the aorta shows the risk of developing infectious complications in coronary heart disease and the importance of dynamic determination of PCT in this case.Thus, the change in the diameter of the aorta depends on the degree of the infectious process in the body. Therefore, in case of CHD, it is important to take into account the state of syntropy, that is, the presence of concomitant diseases, for the prognosis of complications. At the same time, the longer the duration of chronization of the infectious process and the dynamic increase in the level of complement C3, IL-17 and PCT, the greater the risk of an increase in the diameter of the aorta in CHD.It is known that the aorta regulates blood pressure and heart rate. Age-related enlargement of the aortic root leads to thinning of the aortic wall, increases the risk of aortic rupture and/ or aortic aneurysm. Hypertension, hyperglycemia and hypercholesterolemia are risk factors for changes in the state of the vascular wall. At the same time, negative weak connections of the aortic diameter with TNF-a (r= -0.2) and VEGF (r= -0.2) were also revealed. Despite the fact that more than a century has passed since the discovery of TNF-α, its role in the body is still unclear. It is known that this protein causes hemorrhagic necrosis of some tumor cells, hence its name. With the discovery of TNF-α, there was hope that it would be possible to cure cancer with its help. However, further study of the role of this protein disappointed: it turned out that, on the contrary, it can cause cancer, stimulate tumor growth and accelerate metastasis. To date, its multifunctional effect in the body has been established, and the biological effects depend on the concentration of it and its receptors in the blood, their expression [10].TNF-a has the ability to interact with other cytokines and stimulate the secretion of interleukins (IL-1, IL-6, IL-8), INF-γ, chemokines, activates leukocytes during infections, enhances the production of other cytokines [1]. In some cases, it plays a pro—inflammatory role, in others - protective, anti-inflammatory [3,11]. This factor is involved in the pathogenesis of both acute and chronic inflammatory diseases, especially in the elderly, for which it received the name "inflammatory mediator". Its secretion increases in all viral diseases, while it causes the death of cells affected by viruses [6].Consequently, the established negative associations of the aortic diameter with TNF-a in CHD confirm the data of literature sources and allow the inclusion of TNF-a in the list of indicators of the progression of hypertension in CHD as a marker of inflammation in CHD. VEGF is a signaling protein produced by cells to stimulate vasculogenesis and angiogenesis. W. Zheng and co-authors [8] in an experiment, VEGF expression was shown in coronary microvascular endothelial cells (EC) in response to stretch both cardiomyocytes (paracrine pathway) and ECS themselves (autocrine pathway). Various authors have noted the involvement of VEGF in endothelial survival, having an anti-apoptotic effect on the endothelium. A decrease in the level of VEGF causes endothelial apoptosis, leading to obstruction of the vascular lumen. VEGF plays an essential role in the formation and maintenance of vascular lumens - VEGF121 and VEGF165 increase it, while VEGF189 reduces the diameter of the lumen. The protective properties of VEGF also consist in reducing the toxicity of low-density lipoproteins (LDL) in relation to the endothelium [4]. It should be emphasized that the physiological functions of VEGF depend on certain levels of VEGF. It was shown on experimental models that low levels of VEGF have protective properties [7].On the other hand, neoangiogenesis plays an essential role in the transport of cells activated by pro-inflammatory factors into ischemic tissue, as well as in the delivery of nutrition and oxygen [5].Thus, the established connections in our studies allow us to include the conclusion that, along with the above, risk factors for the development of rupture and/ or aneurysm of the aorta in CHD are an increase in the blood level of complement C3, IL-17 and PCT. At the same time, the greater the degree of increase in the dynamics of the level of C3, IL-17 and PCT, the greater the risk of rupture and / or aneurysm of the aorta in CHD. Consequently, the more the level of TNF-a, VEGF increases in coronary heart disease, the more the diameter of the aorta decreases. At the same time, it is necessary to take into account the state of syntropy.In CHD revealed a noticeable positive association of final diastolic volume of the left atrium (FDV LA) with IGF-1 (r=0.30), PCT (r=0.30) and total blood protein (r=0.39) against the background of a weak correlation with TGF-b1-r=0.21 (fig.2). 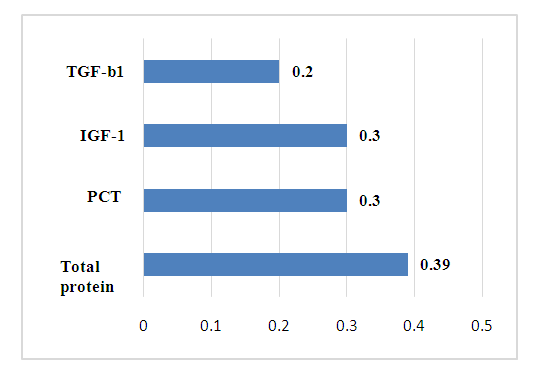 | Figure 2. The relationship of the size of the left atrium with immuno-inflammatory markers in CHD |
The established features of the relationship show the effect of syntropy and chronic inflammation in CHD. At the same time, with coronary heart disease, an increase in the level of TGF-b1 and PCT in the blood against the background of an increase in total blood protein leads to an increase in FDV LA. In CHD, the total blood protein has a noticeable negative relationship with final diastolic volume of the left ventricle (FDV LV) -r =-0.31, which makes it possible to determine it as an indicator of the shift of FDV LA and FDV LV. Consequently, in CHD, an increase in the level of total protein is accompanied by an increase in FDV LA and a decrease in FDV LV. Thus, by determining the total protein in the blood, it is possible to predict the risk of developing LV dysfunction of the heart in CHD.ECHOCG LV indices showed noticeable negative associations between muscle mass of the left ventricle (MMLV) and IL-6-r=-0.31, a high negative association between LVEF and IL-6- r=-0.41. At the same time, final systolic volume of the left ventricle (FSV LV) has a high positive relationship with IL-1- r=0.40, and specific volume of the left ventricle (SVLV) has a high positive dependence on the concentration of C3 in blood serum - r=0.41 (Fig.3).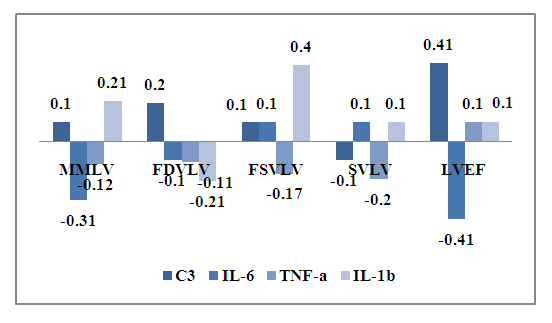 | Figure 3. The relationship of ECHOCG indicators and cytokines in CHD |
Consequently, SVLV decreases with an increase in IL-6, and MMLV increases with an increase in IL-1 in the blood, both cytokines are proinflammatory and show the role of immune inflammation at the level of the heart and blood vessels in coronary artery disease.During the analysis, it was found that the level of SAP directly depends on the concentration of fibrinogen -r= 0.30 and oppositely depends on the concentration of PCT - r =-0.3 and IL-6-r=-0.26.At the same time, a noticeable positive association of heart rate with creatinine in the blood -r=0.35 was also revealed. The established connections show the contribution of inflammation syndrome (immune) in the progression of hypertension, or rather, indicators of the progression of hypertension in coronary heart disease are creatinine, fibrinogen, PCT and IL-6 (Fig.3).Thus, revealed on the basis of correlation analysis of the relationship between the studied blood parameters and functional studies of the heart, the role of inflammation syndrome in the progression of hypertension in coronary heart disease has been established. At the same time, the development of cardiac restructuring dictates the need to develop immunological and biochemical indicators for predicting the progression of hypertension in coronary heart disease in middle-aged and elderly people.The dynamic study of immunological and biochemical blood parameters and the use of these recommendations allows the control and monitoring of hypertension, improving the effectiveness of diagnosis and the correct choice of treatment for patients with CHD, contributes to reducing mortality and disability at the same time. In order to compare the relationship of immunological and biochemical parameters of blood with functional parameters of ECHOCG, a correlation analysis of the results obtained in patients with atypical angina was performed.As a result, aortic diameter connections were established: weak positive with TNF-a (r=0.20), weak negative connections with IL-17A(r= -0.20), total blood protein (r= -0.20), PCT (r= -0.20) and VEGF (r= -0.26).The obtained results of correlation analysis of the relationship allow a comparative assessment with the results of patients with CHD. Distinctive features of the relationship of the studied blood parameters with the diameter of the aorta in coronary artery disease were revealed: there is a weak positive relationship with TNF-a (r=0.20) against the background of a weak negative relationship between the diameter of the aorta and IL-17 (r=-0.20), total blood protein (r=-0.20), PCT (r=-0.20) and VEGF (r=-0.26). Consequently, in the atypical form of coronary angina, there is no risk of rupture and/or aneurysm of the aorta. The study of the relationship between the FDV LA showed a noticeable positive relationship with the blood creatinine level in coronary artery disease (r=0.32).At the same time, weak negative associations of FDV LA with IL-17A (r=-0.21), fibrinogen (r=-0.20) and VEGF (r=-0.20). Consequently, in CHD, an increase in fibrinogen, IL-17A and VEGF in the blood is accompanied by a decrease in FDV LA. The degree of increase in creatinine shows the maximum size of the FDV LA. Therefore, with coronary artery disease, creatinine is an indicator of the prognosis of an increase in FDV LA.In contrast to the correlation between FDV LA in CHD, in atypical angina, a noticeable positive relationship was established between FDV LV and PCT (r=0.30), complement C3 (r=0.40), weak noticeable connections with urea (r=0.20), fibrinogen (r=0.22), total blood protein (r=0.22) against the background of a weak negative association with creatinine (r= -0.21).The results obtained made it possible to determine the C3 complement of indicators of LV dysfunction in coronary artery disease in middle-aged and elderly people.FDV LA in CHD has a high negative association with creatinine (r=-0.47) and TGF-b1 (r=-0.40), a noticeable negative association with IL-6 (r=-0.35), IL-1b (r=-0.30) and total blood protein (r=-0.30), a noticeable positive association with IGF-1 (r=0.30). At the same time, the more creatinine and TGF-b1 increase in the dynamics in the blood, the more the FDV LA decreases. Consequently, creatinine and TGF-b1 are indicators of pancreatic hypertrophy in coronary artery disease atypical angina.Consequently, in atypical angina, a decrease in TNF-a in the blood in dynamics against the background of an increase in fibrinogen, VEGF and IL-1b in the blood indicates a threat of hypertrophy of the LV.The state of the thickness of the posterior LV wall in the diastole (PWLV) is important. There was a noticeable positive association of PWLV with VEGF (r=0.35) against the background of a noticeable negative association with IL-6 (r=-0.37), IL-17A (r=-0.30).At the same time, weak associations of CSL were also revealed: negative with blood urea (r=-0.26) and positive with IGF-1 (r=0.21), which shows a low risk of developing fibrosis in CHD.The data obtained show that with coronary artery disease, an increase in IL-6, C3, and IGF-1 in the blood is accompanied by an increase in the size of the LV and vice versa, a decrease in these indicators in dynamics indicates an increase in LV with atypical angina in middle-aged and elderly people.In general, a correlation analysis of blood parameters in CHD revealed a strong relationship between IL-6 and left ventricle ejection fraction (LVEF) (r=0.44). At the same time, C3 complement and IL-6 were effective informative indicators of the severity of atypical angina (fig.4).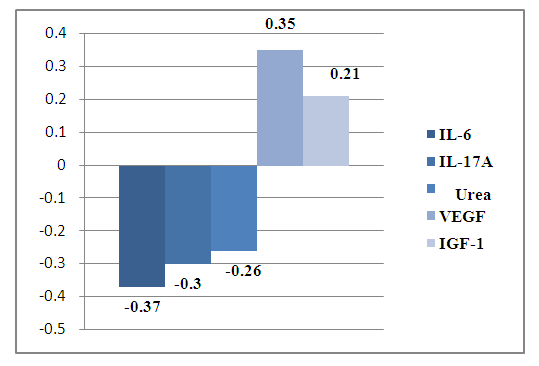 | Figure 4. The relationship of the size of the posterior wall of the left ventricle with immunological parameters in coronary heart disease |
Thus, the obtained high correlations made it possible to determine informative indicators of the risk of cardiac remodeling in CHD: an increase in IL-6 in the blood in dynamics predicts the risk of developing uremia (an increase in creatinine in the blood), tachycardia, a decrease in the thickness of the LV in the diastole, a decrease in LVEF, an increase in MMLV.
4. Conclusions
Thus, the established connections in our studies allow us to include the conclusion that, along with the above, risk factors for the development of rupture and/ or aneurysm of the aorta in CHD are an increase in the blood level of complement C3, IL-17 and PCT. Consequently, with an increase in the level of VEGF in the blood in CHD AS, the risk of an increase in the thickness of the LV in the diastole increases, and an increase in IL-6, IL-17A and urea in the blood shows a decrease in the thickness of the LV in the diastole, which makes it possible to differentiate the restrictive variant from the hypertrophic form in atypical angina. Due to the high and noticeable correlation with the studied immuno-biochemical parameters of blood and functional parameters of the heart, IL-6 is a more informative indicator of the progression of coronary artery disease with the risk of complications and multiple organ failure.
References
| [1] | Akhmaltdinova L., Sirota V., Zhumaliyeva V. et al. Inflammatory Serum Biomarkers in Colorectal Cancer in Kazakhstan Population. Int J Inflam. 2020; 2020: 9476326. DOI: 10.1155/2020/9476326. https://www.rmj.ru/articles/endokrinologiya/Faktor_nekroza_opuholi__i_ego_roly_v_patologii/ #ixzz7wP9ztsD9. |
| [2] | Cypowyj, S., Picard, C., Maródi, L., Casanova, J.-L., and Puel, A. (2012) Immunity to infection in IL-17-deficient mice and humans. Eur. J. Immunol., 42, 2246–2254.] |
| [3] | DeBerge M.P., Ely K.H., Enelow R.I. Soluble, but not transmembrane, TNF-α is required during influenza Infection to Limit the Magnitude of immune Responses and the Extent of Immunopathology. J Immunol. 2014; 92(12): 5839–5851. DOI: 10.4049/jimmunol.1302729. https://www.rmj.ru/articles/endokrinologiya/Faktor_nekroza_opuholi__i_ego_roly_v_patologii/ #ixzz7wP9jpGEP. |
| [4] | Kuzuya M., Ramos M.A., Kanda S. et al. VEGF protects against oxidized LDL toxicity to endothelial cells by an intracellular glutathione-dependent mechanism through the KDR receptor // Arterioscler. Thromb. Vasc. Biol. – 2001. – Vol. 21. – P. 765-770Tsutsumi Y., Losordo D.W. Double face of VEGF // Cir - culation. – 2005. – Vol. 112. – P. 1248-1250. |
| [5] | Osamu I., Matsubara H., Nozawa Y. et al. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs // Circulation. – 2002. – Vol. 106. – P. 2019-2025. |
| [6] | Szretter K.J., Gangappa S., Lu X. et al. Role of Host Cytokine Responses in the Pathogenesis of avian H5N1 Influenza Viruses in Mice. J Virol. 2007; 81(6): 2736–2744. DOI: 10.1128/JVI.02336-06. |
| [7] | Tsutsumi Y., Losordo D.W. Double face of VEGF // Cir - culation. – 2005. – Vol. 112. – P. 1248-1250. |
| [8] | Zheng W., Seftor E.A., Meininger C.J. et al. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGFβ // Amer. J. Phisiol. Heart Circ. Phisiol. – 2001. – Vol. 280. – P. 909-917. |
| [9] | Баширов Н.Х. МАРКЕРЫ ФАКТОРОВ РИСКА РАЗВИТИЯ СЕРДЕЧНО-СОСУДИСТЫХ ЗАБОЛЕВАНИЙ // ЕКЖ. 2020. №3. URL: https://cyberleninka.ru/article/n/markery-faktorov-riska-razvitiya-serdechno-sosudistyh-zabolevaniy. |
| [10] | Терещенко И.В., Каюшев П.Е. Фактор некроза опухоли α и его роль в патологии. РМЖ. Медицинское обозрение. 2022; 6(9): 523-527. DOI: 10.32364/2587-6821-2022-6-9-523-527. |
| [11] | Тополянская С.В. Фактор некроза опухоли альфа и возрастассоциированная патология. Архив внутренней медицины. 2020; 10(6): 414–421. DOI: 10.20514/2226-6704-2020-10-6-414-421. |
| [12] | Тугуз А.Р., Шумилов Д.С., Муженя Д.В., Лысенков С.П., Смольков И.В., Татаркова Е.А., Хацац Д.З., Ашканова Т.М. ДИСБАЛАНС СУБПОПУЛЯЦИЙ NK-КЛЕТОК И ПОЛИМОРФИЗМЫ ГЕНОВ ПРОВОСПАЛИТЕЛЬНЫХ ЦИТОКИНОВ В ПАТОГЕНЕЗЕ АТЕРОСКЛЕРОЗА // Медицинская иммунология. 2022. №1. URL: https://cyberleninka.ru/article/n/disbalans-subpopulyatsiy-nk-kletok-i-polimorfizmy-genov- provospalitelnyh-tsitokinov-v-patogeneze-ateroskleroza. |
| [13] | Шилова Л. Н., Паньшина Н. Н., Чернов А. С., Трубенко Ю. А., Хортиева С. С., Морозова Т. А., Паньшин Н. Г // Журнал Современные проблемы науки и образования. – 2015. – № 6. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML