-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(5): 569-575
doi:10.5923/j.ajmms.20231305.05
Received: Apr. 6, 2023; Accepted: May 5, 2023; Published: May 12, 2023

A New Composite Hemostatic Plate in Parenchymal Organ Surgery
B. R. Abdullajanov, A. Kh. Babajanov, R. A. Sadikov, M. Y. Kuchkarov
Andijan State Medical Institute, Andijan, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to improve the results of stopping parenchymal bleeding in abdominal surgery by developing and clinically evaluating the efficiency of a new domestic composite hemostatic plate.The article presents the experimental and histomorphological studies results of the use of composite hemostatic plate. A pronounced hemostatic property of the plate in the vessels in the wound area was proved by the results of the study. Due to the fact that inflammatory reactions in the wound area are predominantly proliferative in nature, hemostasis plate restores the histoarchitectonics of wound tissue in the wound area in the early stages and prevents rough scarring due to the lack of a response to foreign bodies, prevents the occurrence of complications.
Keywords: Parenchymal organ, Bleeding, Hemostatic plate
Cite this paper: B. R. Abdullajanov, A. Kh. Babajanov, R. A. Sadikov, M. Y. Kuchkarov, A New Composite Hemostatic Plate in Parenchymal Organ Surgery, American Journal of Medicine and Medical Sciences, Vol. 13 No. 5, 2023, pp. 569-575. doi: 10.5923/j.ajmms.20231305.05.
1. Introduction
- Bleeding and hemorrhagic shock are the cause for 30-40% of traumatic deaths. Besides, perioperative and examination-related procedures may lead to a risk of bleeding [8,10]. Electrocoagulation, argon, microwave, laser and radiofrequency coagulation, contact infrared radiation, ultrasonic, harmonic, jet scalpels, plasma streams, radiofrequency ablation are used among the physical methods of hemostasis in surgery of parenchymal organs. Achieving a hemostatic effect by physical methods of influencing the wound surface and bleeding vessels of the parenchyma is rational mainly with its shallow and superficial injuries [6]. An increase in exposure and energy exposure is required to stop bleeding from parenchymal vessels with a diameter of more than 1.0 mm, which inevitably leads to the injury of the organ stromal elements and increases the area of parenchymal necrosis to a depth of 4-8 mm, and the coagulation scab formed in this case often serves as a substrate for infection and recurrence of bleeding [7]. Physical methods of hemostasis during surgeries on parenchymal organs do not meet the requirements of the "ideal method", which should be accompanied by minimal blood loss or its absence, minimal necrosis of the parenchyma and a reduction of the suregry time [4]. A study of surgeons’ opinion conducted by Bondarev et al. (2020) on the use of local hemostatic agents showed that 30% of 135 doctors of various surgical specialties resorted to hemostatic sutures and 27% used local hemostatics, splenectomy was performed in 13%, electrocoagulation in 18% [2]. Particular attention is paid to issues related to indications for the use of local hemostatic agents. According to 50% of respondents, local remedies were used as the main method of stopping bleeding in case of parenchymal organ injury of the first degree. In cases of injuries of I-III-degree, local hemostatics can be used as an additional method of stopping bleeding. At the same time, 42% of surgeons believe that hemostatic suture should be covered with hemostatic material from above after its application; 26% placed the implant under the suture, and then tied a knot on it; 10% believe that it is necessary to place a hemostatic in the wound, and 22% of respondents believe that it makes no sense to combine the use of a local hemostatic agent with a hemostatic suture of a parenchymal organ.The final part of the questionnaire was devoted to assessing the opinion of surgeons on what should be the ideal local hemostatic agent. Как показал опрос, 95% of respondents believe that the hemostatic material should have such properties as antibacterial activity, non-pyrogenicity 60.9%, high adhesive ability 58.6%, biodegradation within 5-10 days after implantation 36.1%, hemostatic effect within 2 min after application 60%, 5-10 min 27%. At the same time, 84% of respondents believe that the speed of stopping bleeding is not significant, the main thing is the reliability of stopping bleeding [2].The use of hemostatic agents is essential to prevent serious bleeding and death from excessive bleeding during surgery or in emergency situations. Cellulose oxide is an excellent biodegradable and biocompatible derivative of cellulose and has become one of the most important hemostatic agents used in surgical procedures. There is still no comprehensive report on the evaluation of cellulose-based hemostatic substances, although the preparation for oxidation, the origin and structure of cellulose, as well as the biodegradability and safety of cellulose oxide have been considered. Hemostatic mechanisms of various forms, variations and currently commercially available cellulose products are comprehensively discussed, which highlights the most important development in the recent scientific literature [1,3,5,9,11].The aim of the study was to improve the results of stopping parenchymal bleeding in abdominal surgery by developing and clinically evaluating the efficiency of a new domestic composite hemostatic plate.
2. Material and Methods
- In previous studies, the entire range of necessary preclinical researches was carried out in the experimental department of the Republican Specialized Scientific and Practical Medical Center of Surgery named after V. V.Vakhidov, the results of which allowed to substantiate the hemostatic efficiency of the new domestic implant in the form of a plate, taking into account the determination of the basic physical and chemical properties and the proven absence of toxic effects (according to the requirements for conducting studies of similar composite materials). The optimal composition of a biodegradable hemostatic plate was developed and experimental studies were conducted on small experimental animals (rats). This article presents the results of the second series of experimental studies on large animals (dogs, pigs). The results of experimental and morphological studies allowed to obtain the permission of the Ethics Committee to conduct the first clinical trial, followed by the preparation of all necessary documentation for the registration of a new domestic hemostatic implant in the Pharmaceutical Committee of the Republic of Uzbekistan with the possibility of subsequent serial production.This study was conducted to evaluate the hemostatic effect of the new plate coating on large-sized liver defects (experimental liver injury in large animals). This will allow us to determine the efficiency of the claimed properties of the implant in extensive areas of parenchymal bleeding. Hemostatic efficacy in spleen injuries was also additionally studied. Currently, the evaluation of the medicines’ efficiency and in particular, hemostatic agents, is carried out on small laboratory animals. These experiments are easy to model, low-cost and allow to trace the dynamics of the pathological process and evaluate the efficacy of treatment. However, in real conditions it is impossible to fully reflect the peculiarity of hemostasis which takes place in clinical practice. In this regard, for the development of recommendations and a real assessment of the efficacy of hemostatic agents, the most adequate model is an experiment on large experimental animals. Mongrel dogs are used for this purpose, as well as recently mini pigs. The mini-pig is an ideal model for evaluating the efficiency of hemostatic drugs, since they correspond to a human in many functional and anatomical parameters.The objective of these studies was to develop a model of parenchymal bleeding in large animals (dogs, pigs), as well as to evaluate the efficiency of the domestic coating – composite plate.Course of the surgery: Hemostasis in case of liver injury. After removing the coat from the anterior abdominal wall, an upper-middle-median laparotomy was performed. After dilution of the wound edges with a retractor, the right lobe of the liver was removed into the surgical field (Fig. 1). Using an abdominal scalpel, the liver parenchyma up to 3x4 cm in area was injured with the development of mixed bleeding (Fig. 2).
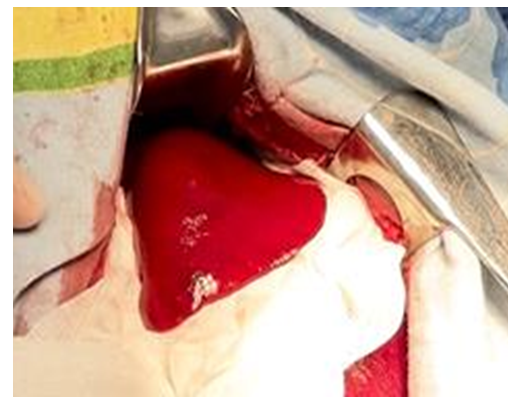 | Figure 1. Simulation of liver injury in the experiment. The right lobe of the liver was brought into the wound |
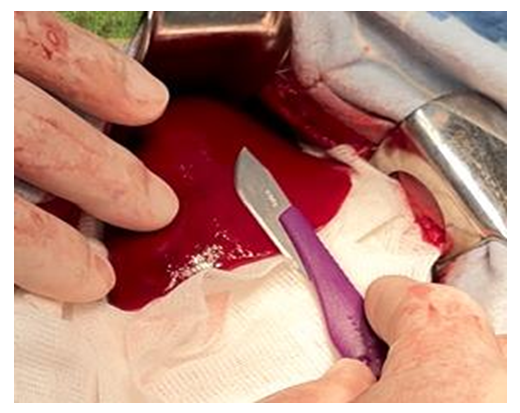 | Figure 2. Liver wound formation |
3. Results
- Control. A hemostatic sponge made of bovine collagen manufactured by "Turon Silk Pharm" LLC is a porous plate of light-yellow color. To stop bleeding from the liver, its fragment 3x4 cm in size was used. After drying wound surface of the liver, the sponge was immediately applied and pressed against the wound (Fig. 3). The sponge was held by hand for 2 minutes. Subsequently, due to the lack of adhesive ability, the sponge was kept on the liver surface by tamponing with a gauze napkin (Fig. 4).
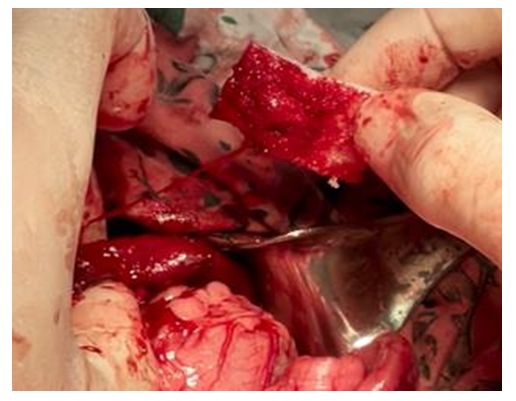 | Figure 3. Partial hemostatic effect of collagen sponge |
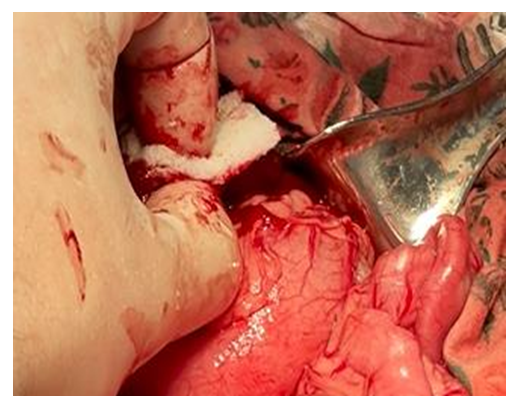 | Figure 4. Hemostasis of the liver wound surface with a hemostatic sponge |
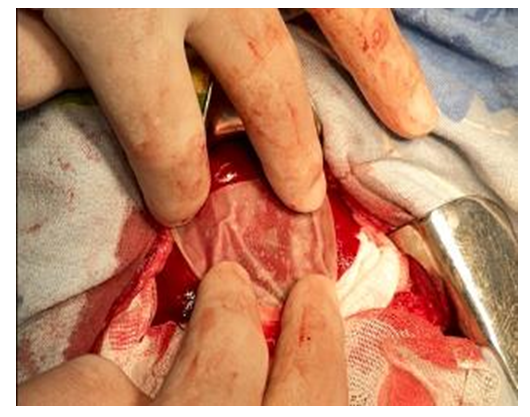 | Figure 5. Experiment. The moment of applying the hemostatic plate to the wound surface of the liver |
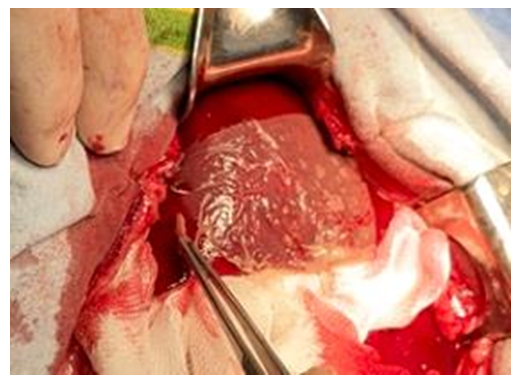 | Figure 6. Experiment. An attempt to remove the hemostatic plate from the surface of the liver wound. The plate does not come off from the wound when pulled up with tweezers |
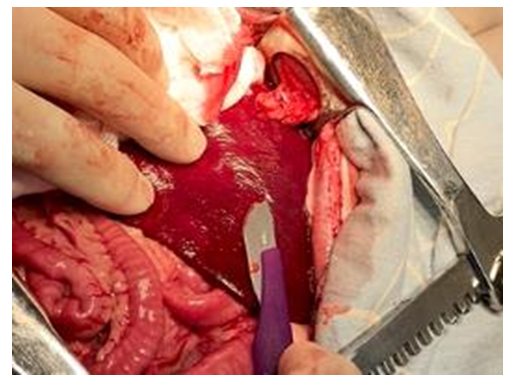 | Figure 7. Simulation of the spleen injury. A planar wound of the spleen is formed using an abdominal scalpel |
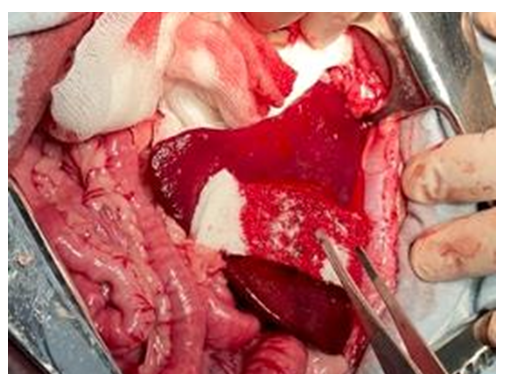 | Figure 8. Control. The result after repeated application of the hemostatic sponge to the spleen wound surface. Final hemostasis was not achieved |
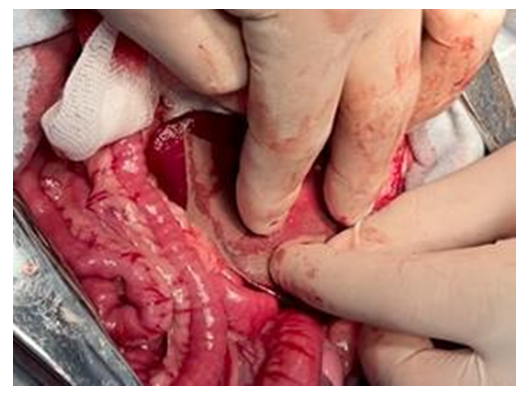 | Figure 9. The moment of applying the hemostatic plate to the spleen wound surface |
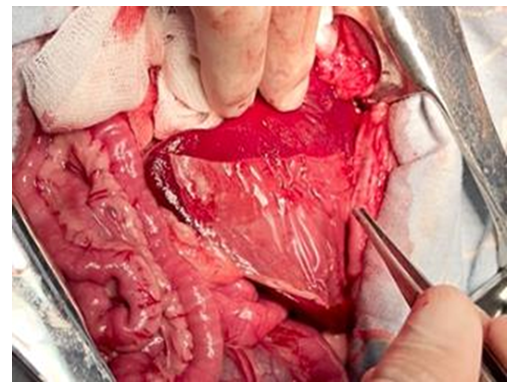 | Figure 10. Experiment. A complete adhesion of the hemostatic plate to the spleen wound surface with final hemostasis was after 30 minutes |
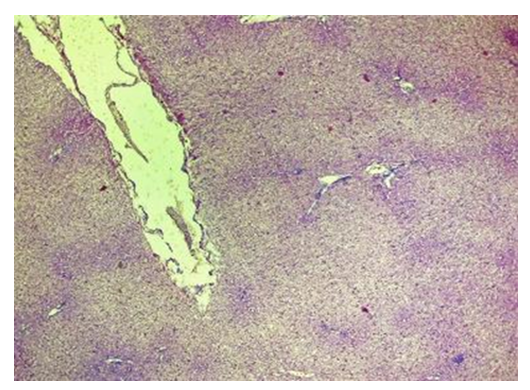
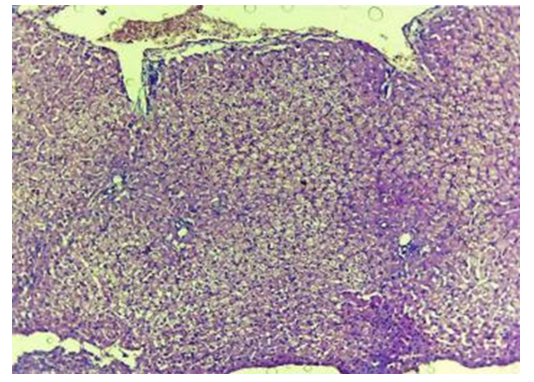 | Figure 12. Liver with a contact zone of PHP exposure. Stasis and sludge-state of red blood cells. Blood clots in the PHP area. The 3rd day of experience. CM. Stain with H-E х100 |
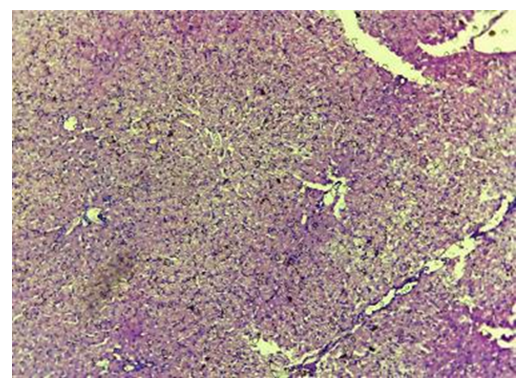 | Figure 14. Control. 30 days. Fragments of stacks of oxidized cellulose are encapsulated. The fibers are indistinguishable. CM. Stain with H-E х100 |
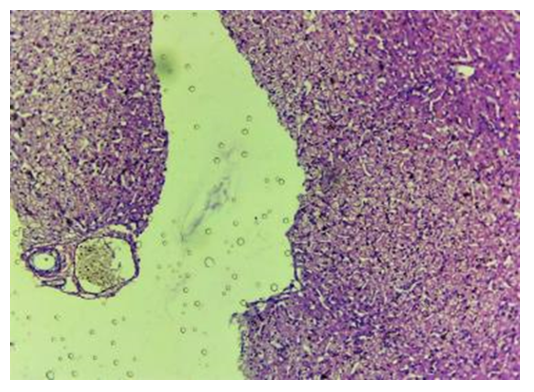
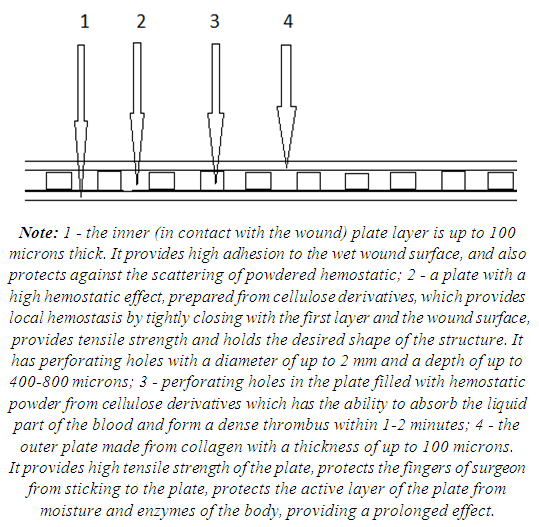 | Figure 15. Schematic structure of a composite plate for hemostasis and tissue strengthening |
4. Conclusions
- The conducted experimental studies allowed to develop the first domestic composite hemostatic plate for the use in parenchymal organs surgery, which includes several layers based on biologically absorbable polymer derivatives (cellulose, viscose and calcium), the combination of which provides local hemostatic efficiency of the implant. Due to its properties, the use of a composite hemostatic plate in the wound area causes rapid histoarchitectonic remodeling of tissues in the area of contact with the organ. The plate after topical application does not cause a strong tissue and response reaction as a foreign body, which makes it biocompatible. Ca2+ ions in the polycomposite structure of the implant have a direct and indirect effect on the blood clotting process, which in turn provides a stable hemostatic property on the wound surface.The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML