-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(4): 475-478
doi:10.5923/j.ajmms.20231304.25
Received: Apr. 2, 2023; Accepted: Apr. 20, 2023; Published: Apr. 22, 2023

Morphogenesis and Morphology of Stromal Structures in Glandular Hyperplasia of the Prostate Gland
Khamraev O. A.1, Israilov R. I.2, Kosimhojiev M. I.1
1Andijan State Medical Institute, Andijan, Uzbekistan
2Republican Center of Pathological Anatomy, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The main of this studyThe aim was to clarify the morphogenesis and pathomorphological changes of stroma tissue structures in prostate glandular hyperplasia. It has been confirmed that the disease of prostatic hyperplasia consists of 5 morphologically stages. In the first stage of the disease, it is observed that 2 or 3 glandular acini are hyperplastic separately, forming a single unicentric micro-nodule, and the connective tissue between them is roughened and increased. Starting from the second period of the disease, lympho-plasmacytic cells first increase in the stroma, then after the proliferation of histiocytic cells, the inflammatory infiltrate densely surrounds the gland cells and forms a membrane. Exacerbation of the inflammatory process in the stroma of the gland is acidification of the tissue secretion in terms of biochemical composition, on the other hand, it is the development of an autoimmune process against the cells of the gland cells and stroma tissue structures.
Keywords: Prostate, Hyperplasia, Stroma, Morphogenesis, Morphology
Cite this paper: Khamraev O. A., Israilov R. I., Kosimhojiev M. I., Morphogenesis and Morphology of Stromal Structures in Glandular Hyperplasia of the Prostate Gland, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 475-478. doi: 10.5923/j.ajmms.20231304.25.
1. Introduction
- When discussing morphological changes in the disease of safe hyperplasia of the prostate, one must first call the processes correctly and with specific terms. An increase in the volume of organelles, tissue, cell or intracellular, is called hypertrophy in General Pathology. The numerical reproduction of the organ and tissue component is called hyperplasia. In the study of the skirts of the morphogenesis of hyperplastic processes in prostate-safe hyperplasia, it will be necessary to take into account the presence of parenchyma consisting of glandular structures in the tissue of this member and fibromuscular stroma between them [1,2,3]. Anatomically, the size of the organ increases from the appearance of nodular structures in the structure of the gland, and the development of this process can consist of several periods. At the beginning of the process, nodes appear from 2-3 densely packed glands. Around these glands, the myofibrillary stroma develops into coarsened connective tissue, giving rise to a distinctive fibrosis curtain. Later in the development of the process, the glandular ayinar structures hyperplasia and increase dramatically, and microscopically the appearance of numerous nodules coincides with period II. Around these glandular nodes, stroma tissue structures proliferate, creating connective tissue curtains. The outbreak of the pathological process produces new additional specific proliferative centers around the glandular nodes and is a morphological appearance of period III. An active increase in asinar foci leads to the dimming of secretions from them and the formation of retentive cysts in asinuses, and this is the IV-period of the process. The epithelium covering the asinuses that have become cysts is the V-Day of the process if it atrophy and becomes smaller in shape. Prostate safe hyperplasia paralleling developmental morphogenetic periods, stroma structures also proliferate, proliferate, and outperform parenchyma in volume when it comes to the last period, and this is known as fibrosed adenosis [4,5,6,7,8]. Asynar yachies are fragmented as a result of proliferation and proliferation of fibromuscular structures in the gland stroma. The main goal of this study was considered to clarify the morphogenesis and pathomorphological changes of stroma tissue structures in glandular hyperplasia of the prostate.
2. Material and Methods
- As an object of examination, the men's prostate in the safe hyperplasia of the prostate, which was examined in the direction of biopsy diagnostics of the Republican Center for pathological anatomy, the prostate fragments obtained in the type surgery were hardened for 48 hours in 10% solution of formalin for histological examination purposes. After 4 hours of washing in running water, the concentrate was dehydrated in increasing alcohols and paraffin was poured and bricks were prepared. 4-5 µm histological incisions were made from paraffin bricks and painted in the hematoxylin-eosin and Van-Gison methods. The preparations were examined under a light microscope and the desired areas were photographed.
3. Results and Discussion
- In the i-period of safe glandular hyperplasia of the prostate, it is found that the 2nd or 3rd glandular asinuses hyperplasia separately, giving rise to a single unisentric micro node. This gland begins to proliferate, proliferating fibrocytic and myocytic cells, as well as fibrous structures, containing stroma between and around the yachts. The specificity of proliferation of these tissue structures is that fibrocytes become fibroblasts, with increased proliferative activity causing their nuclei to both hyperchromasize and increase in size. Fibroblasts have produced large amounts of fibrous structures and are found to have produced tufts of varying sizes from joining with each other, and they wrap the glandular yachts densely. Affected by the proliferation process of fibroblasts, smooth muscle cells between them also proliferate and proliferate (Figure 1), and are found to join fibrocytic tissue Tufts to form combinatorial fibromuscular tissue and wrap the glandular sacs. In the conclusion, it can be shown that from a violation of hormone metabolism, initially, the glandular structures of the prostate parenchyma undergo hyperplasia, and next, from the fact that hormones also affect the stroma tissue structures, the fibrocytic and myositar cells in them proliferate, fibromuscular tissue structures with a coarse structure are formed from the production of fibrous structures.
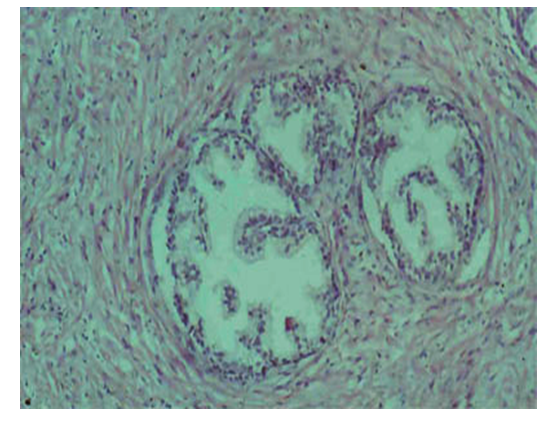 | Figure 1. Hyperplasia of individual glandular yachts in the prostate and surrounded by Stroma tissue. Paint: G-E. Size: 10x40 |
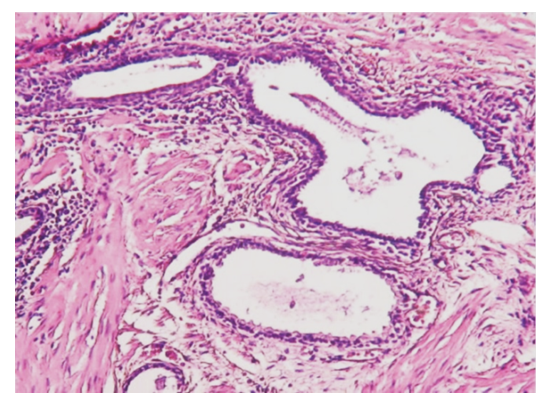 | Figure 2. The appearance of inflammatory infiltrate in the hyperplasia and stroma of the asinus of the prostate gland. Paint: G-E. Size: 10x40 |
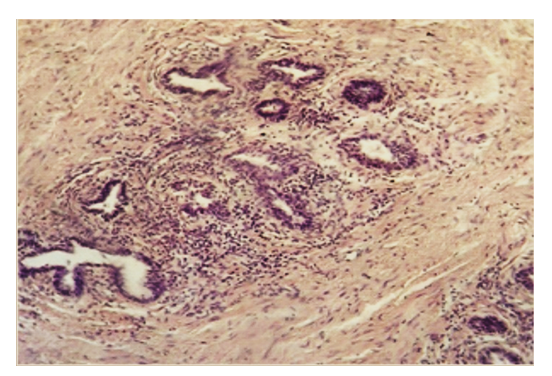 | Figure 3. Increased inflammatory process in stroma in glandular hyperplasia of the prostate. Paint: G-E. Size: 10x40. |
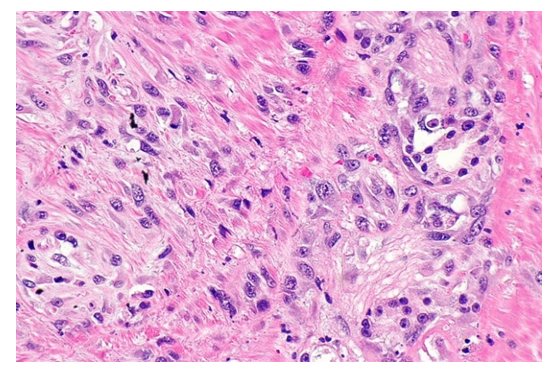 | Figure 4. Activation of prostate stroma cells and fibrous structures. Paint: G-E. Size: 10x40 |
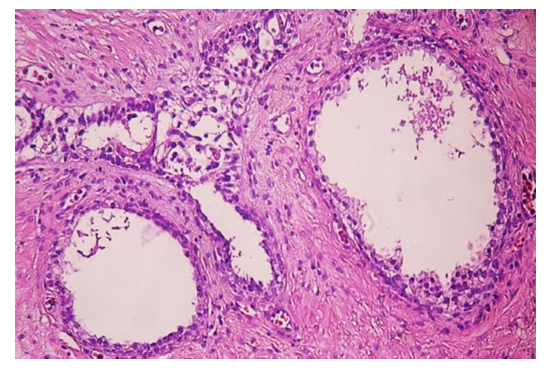 | Figure 5. The transformation of glandular asinuses into cysts, the growth of coarse fibrous fibromatous tissue in the intermediate tissue. Paint: G-E. Size: 10х40 |
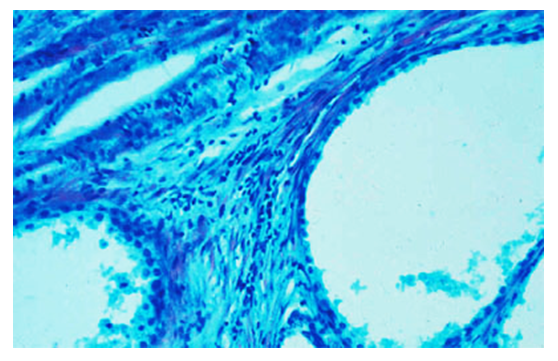 | Figure 6. An increase in sour mucopolysaccharides in the gland stroma, the appearance of Musin and muciod in the cavity of ksita. Paint: Kreyberg method. Size: 10х40 |
4. Conclusions
- It has been confirmed that prostate glandular hyperplasia consists of 5 periods that develop morphologically one after another.In the I-period of the disease, it is observed that the 2nd or 3rd glandular asinuses hyperplasia separately, giving rise to one unisentric micro node, the connective tissue between which becomes rough and increases. Since the II-period of the disease, stroma initially increases lympho-plasmocytic cells, then after the addition of proliferation of histiocytic cells, inflammatory infiltrate envelops the glandular cells densely, creating a veil.The advance of the inflammatory process in the gland stroma is the sourness of the tissue secretion in terms of its biochemical composition, but on the other hand it is the development of the autoimmune process in relation to the cells of the glandular cells and the tissue structures of the stroma.In glandular hyperplasia of the prostate, the low-specific fibroblasts contained in the activation of the stroma increase actively, differentiated fibroblasts produce fibrous structures, glycosaminglicans and intermediate, on the other hand, monocytes become large histiocytes, phagocytosis of foreign bodies, co-operationalization with humoral immune cells, lysozyme and interferon synthesis, and synthesis of polynuclear leukocyte desecration factor synthesis. As a result, anomalous collagen is synthesized, they undergo degradation, collagen fiber composition and structure are disrupted, elastic fibers are formed and added with collagen, the destruction of fibrous structures is caused by the development of an autoimmune process.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML