Azizova G. D., Asatova M. M., Dauletova M. J.
Republican Specialized Scientific and Practical Medical Center for Obstetrics and Gynecology, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this article, the subjects were divided into groups: group I included 215 women with ovarian hyperandrogenism, group II included 72 women with adrenal hyperandrogenism III- the control group included 30 women. LH indicators in all patients with HA were significantly higher than those in the control group, while FSH indicators in both groups were normal, however, the ratio of the LH/FSH index was 3.04±0.58 in the first group, in the second group it was 2.19 ± 0.89 (p=0.07), AMH indicators were almost 2.5 times higher in group I, than those in group II. It has been found that a direct dependence of the AMH level with an increase in the level of androgens in the blood, an increase in the volume of the ovaries and the number of follicles in them, with an AMH level of more than 8.2 ng/ml, in combination with the presence of echographic signs of multifollicular ovaries, can be regarded as the formation of polycystic ovary syndrome. In group I, the median value of inhibin B was 2 times higher than that in the control group (p=0.0009) and in group II it was 1.4 times higher (p=0.004). There was an increase in the level of inhibin B in patients with ovarian dysfunction, clearly correlated with both the level of AMH and the size of the follicles. In addition, in group I there was a significant direct correlation of inhibin B with the level of LH and the ratio of LH/FSH, which proved the LH-dependent nature of the increase in inhibin B in this category of patients.
Keywords:
Infertility, Hyperandrogenism, Menstrual disorder, AMH, Inhibin B
Cite this paper: Azizova G. D., Asatova M. M., Dauletova M. J., Studying the Features of Intraovarian Mechanisms of Regulating Reproductive Function in Women with Hyperandrogenism of Various Genesis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 463-466. doi: 10.5923/j.ajmms.20231304.22.
1. Introduction
In recent decades, despite the increase in fertility and life expectancy, the country has seen a deterioration in the health of women of fertile age. It is known that one of the significant causes of dysfunction and diseases of the reproductive system are hyperandrogenic conditions, the frequency of which, according to various authors, ranges from 4 to 18% [1,2,10]. Restoration of reproductive function in patients with various forms of hyperandrogenism is an urgent medical and social problem. Hyperandrogenism is one of the difficult−to-diagnose pathologies of the endocrine system in women of reproductive age [2]. In most cases, the pathology is formed during puberty, however, in a number of patients this condition may be observed in an earlier period [3]. The lack of clear boundaries between norm and pathology causes a lot of controversy. It is necessary to conduct further studies aimed at clarifying molecular genetic factors, intraovarian markers, and pathogenetic mechanisms for the development of anovulation. The intra-ovarian mechanisms of disruption of the processes of initiation of the dominant follicle and ovulation remain poorly understood. Further study of the relationship between intraovarian markers and the central mechanisms responsible for folliculogenesis is required. Of particular scientific and practical interest is the study of the role of peptide hormones of AMH and inhibin B in the mechanisms of reproductive function disorders depending on the genesis of hyperandrogenism. The study of the pathogenetic mechanisms of the formation of reproductive function disorders depending on the genesis of HA will make it possible to develop effective protocols for the restoration of fertility.The purpose of the study. To study the features of endocrine mechanisms of formation of reproductive function disorders in women with hyperandrogenism, depending on the genesis.
2. Materials and Methods of the Research
The study involved 317 women aged 18 to 35 years with clinical signs of HA and reproductive dysfunction, who came to the consultative clinic of Republican Specialized Scientific and Practical Medical Center for Obstetrics and Gynecology. The data of the anamnesis of the disease, the nature of clinical signs of HA and the peculiarities of the formation of the menstrual cycle, ultrasound examination of the uterus, ovaries, thyroid gland and hormonal examination (prolactin, LH, FSH, TSH, Anti-TPO, T4, 17-OH-progesterone, DHEAS, free testosterone, AMH and inhibin B) were analyzed.We performed an ultrasound examination on a modern ultrasound machine of the expert class Mindray DC-70 with a sensor sensitivity of 7.5 MHz, preference was given to transvaginal access. The patients with regularly menstruating were examined in the early follicular phase (3-5 days of the cycle), and patients with opso-/amenorrhea - on the day of coming or on days 3-5 of induced bleeding after the progestogen test. The follicles were counted in the longitudinal, transverse and anteroposterior sections of the ovaries, the volume of the ovaries, the average size of the follicles, measured in three sections, and the index of the ratio of the area of the stroma to the area of the ovary were observed. The hormonal study was carried out on an enzyme immunoassay analyzer Mindray 96 MR-96A China 2014. Statistical processing of the research results was carried out by common methods using a personal computer, programs Microsoft Word 2016, Microsoft Excel.
3. Results and Discussion
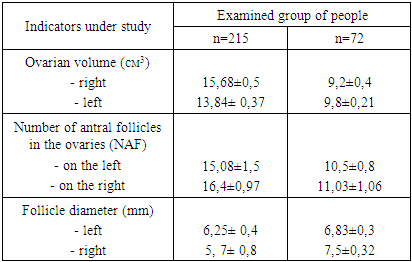
To achieve the objectives of the study, the subjects were divided into clinical groups: group I included 215 women with ovarian hyperandrogenism (PCOS)- the diagnosis was established based on the ESHRE/ASRM consensus criteria [7], in group II there were 72 women with adrenal hyperandrogenism, who had elevated DHEA-C and 17-OH-progesterone (to confirm the adrenal origin of androgens, we conducted a test with dexamethasone), III- the control group included 30 women who came to the consultative clinic of Republican Specialized Scientific and Practical Medical Center for Obstetrics and Gynecology in order to undergo a preventive examination.Currently, ultrasound examination (ultrasound) using a transvaginal sensor allows getting a clear idea of the internal structure of the ovaries, assess the state of the stroma and follicular apparatus (see Table 1). For a detailed study of the structure of the ovaries, all the studied women underwent ultrasound examination.Table 1. The results of the assessment of ultrasound parameters of the ovaries
 |
| |
|
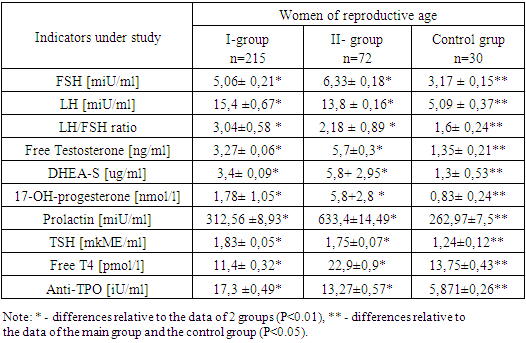
In patients from group I, a significant increase in ovarian volume was found due to hyperechoic stroma, the average volume of the left ovary was 15.68 ± 0.5 cm3, the volume of the right ovary 13.84 ± 0.37 cm3 (normally 8.6 ± 0.3 cm3); with multiple follicles, an average of 15.08±1.5 pcs on the left, 16.4±0.97 pcs on the right; 2-9 mm in diameter and averaged 6.25 ± 0.4 mm on the left, 5.7 ± 0.8 mm on the right, located along the periphery of the ovaries. The total area of the stroma was 2.4±0.6 cm2. At the same time, in women from group II, a slight increase in ovarian volume was observed; the average ovarian volume was 9.2±0.4 cm3 on the left and 9.8±0.21 cm3 on the right, the number of follicles did not exceed 8-10 pcs, it averaged 10.5±0.8 pcs and 11.03±1.06 pcs on the right, with a follicle diameter of 4-12 mm, on average on the left 6.83± 0.3 and on the right 7.5±0.32 of the cellular structure. The results of counting the antral follicles showed that in 227 patients (40.8%) the number of antral follicles (NAF) and the volume (V) of the ovary are larger, on average, the NAF is 18.08 ± 1.5 pcs and V is 14.76 ± 0, 43 cm3, and in 119 patients (21.4%) NAF is 10.5 ± 0.8 pcs and V is 9.5 ± 0.3 cm3.The next stage of the research was the analysis of hormone levels depending on the genesis of HA (Table 2).Table 2. The results of determining the content of gonadotropins, TSH, prolactin and steroid hormones of the ovary
 |
| |
|
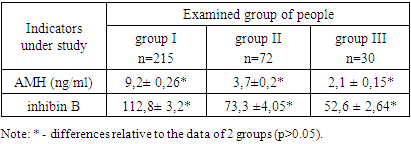
The results of hormonal studies indicate a slight increase in the level of LH in both groups compared with the control group and averaged 15.4 ± 0.67 mIU / ml in group I, 13.8 ± 0.16 mIU / ml in group II, FSH values were within the normal range in both groups and averaged 5.06 ± 0.21 mIU / ml and 6.33 ± 0.18 mIU / ml, while the ratio of the LH / FSH index in group I was 3.04 ±0.58, in group II it was 2.18 ± 0.89.In the women from group I, the concentration of free testosterone averaged 3.27 ± 0.06 ng / ml, while in the patients with adrenal HA, a moderate increase up to 5.7 ± 0.3 ng / ml in indicators was observed compared to the control group. An increase in testosterone levels is an uninformative criterion for determining the source of HA. The concentration of DHEAS in the blood of the women in group I averaged 3.04±0.09 ug/ml, in group II it was higher compared to the control group and averaged 5.8+2.95 ug/ml. The level of 17-OH-progesterone in group I averaged 1.78 ± 1.05 nmol/l, in group II it was higher compared to the control group and averaged 5.8+2.8 nmol/l. Biochemical criteria for adrenal hyperandrogenism today is considered to be an increase in blood concentrations of 17-OHP [8]. The level of prolactin in the first group was normal compared to the control group and averaged 312.56 ± 8.93 mIU / ml, however, in the second group it was 2.4 times higher compared to the control group and averaged 633, 4±14.49 mIU/ml, which is typical for HA of adrenal origin [8].As for the thyroid status, the level of thyroid-stimulating hormone in the studied patients was normal, in group I it averaged 1.83±0.05 mIU/ml, in group II it was 1.75±0.07 mIU/ml. Free T4 levels were within normal limits in group I, 11.4 ± 0.32 ng/ml and in group II, 22.9 ± 0.9 ng/ml. The level of Anti-TPO averaged 17.3 ± 0.49 iU/ml in group I, and 13.27 ± 0.57 iU/ml in group II, that is, it was within the normal range.Analysis of the results of the study of gonadotropin levels showed that in all the patients with HA, LH concentrations were significantly higher than those in the control group. This fact indicates that, regardless of the cause of HA, most patients have signs of impaired gonadotropic, and therefore, ovarian function [9]. A change in the secretion of gonadotropins can be primary as a result of a distortion of circoral secretion of gonadotropin-releasing hormone or secondary - in response to a distortion of ovarian steroidogenesis according to the principles of reverse afferentation. At the same time, in the patients with ovarian HA, the biochemical criteria for PCOS were revealed - an increase in the LH / FSH ratio, which was 3.04 ± 0.58, with adrenal hyperandrogenism, the ratio was 2.19 ± 0.89. In the patients with adrenal HA, the levels of testosterone and 17-OHP were significantly higher than those in the group of patients with ovarian HA and the control group (p>0.05).The next stage of the research was the study of the intra-ovarian mechanisms of the formation of menstrual disorders and reproductive dysfunction by studying the level of peptide hormones AMH and inhibin B in women with HA and reproductive dysfunction depending on the genesis of hyperandrogenism (Table 3).Table 3. The results of determining the content of AMH, inhibin B
 |
| |
|
Currently, it is relevant to search for informative markers for the differential diagnosis of reproductive function disorders in patients with HA, depending on the genesis. Along with the study of the central mechanisms of regulation of folliculogenesis, much attention is paid to intra-ovarian factors. One of the significant mechanisms of regulation of folliculogenesis determining the growth and differentiation of follicles is inhibin B, AMH.The full function of the ovaries is defined by the concept of ovarian reserve, that is, the ability of the ovaries to adequately respond to pituitary stimulation by the growth of full-fledged follicles [12]. High levels of androgens inhibit the maturation of follicles at different stages and lead to the formation of enlarged ovaries with many follicles. In this regard, AMH plays an important role in folliculogenesis [11,12].As follows from these tables, the AMH values are almost 2 times higher in group I than those in group II and it averages 9.2 ± 0.26 ng/ml and 37 ± 0.2 ng/ml, respectively. In group I, AMH correlated with the number of small and medium follicles (R=0.65, p=0.03), while in group II and in the control, no such relationship was observed. We have found a significant connection and dependence between the increased level of blood androgens, the level of AMH and the echographic features of the ovaries, in particular, an increase in their volume and the number of follicles.Thus, at an AMH level of more than 8.2 ng / ml, in combination with the presence of echographic signs of multifollicular ovaries, it can be regarded as the formation of polycystic ovary syndrome.When analyzing the level of inhibin B in the patients with HA, the indicators of inhibin B in group I averaged 112.8 ± 3.2 pg/ml, in group II it was 73.3 ± 4.05 pg/ml. An increased level of this indicator was revealed in comparison with the control group, although in general, its indicators in all examined corresponded to the official reference limits. At the same time, in the subjects of group I, the median of inhibin B was 2 times higher than that in the control group (p=0.0009) and 1.4 times higher than that in the II clinical group (p=0.004). In the patients from group I, the level of inhibin B positively correlated with the level of FSH (R=0.42; p=0.03), inverse correlations were established between the level of inhibin B and the level of 17-OP (R=-0.42; p=0.06).Despite the large ovarian volume and increased follicle count commonly found in women with polycystic ovary syndrome (PCOS), many studies have shown that inhibin B is not elevated as it would be expected in PCOS [5,6]. At the same time, in our study, we found a certain increase in the level of inhibin B in patients with ovarian dysfunction, which clearly correlated with both the AMH level and the size of the follicles. An increase in the level of inhibin B in women with PCOS is also found by other authors. In addition, in group I, there was a significant direct correlation of inhibin B with the level of LH and the ratio of LH / FSH, which proved the LH-dependent nature of the increase in inhibin B in this category of patients. A similar relationship between inhibin B and LH has been shown in some other studies in adult women with PCOS [4,6]. These facts prove the involvement of the impaired secretion of inhibin B in the formation of PCOS, and some inconsistency in the data may be due to the supposed pulsatile nature of its secretion, which is disturbed during the formation of PCOS. In the group of patients with ovarian dysfunction, in contrast to patients without it, a positive correlation of inhibin with androgens was noted, indicating a direct relationship between an increase in the number of immature follicles in the ovarian tissue and an increase in androgen levels. Therefore, although the level of inhibin B is not as indicative of the formation of PCOS as AMH, it should be recognized that changes in its secretion and disturbances in the normal connection with gonadotropins occur already in the early stages of the development of ovarian dysfunction in women with hyperandrogenism. This may be an important pathogenetic link in the development of PCOS in this category of patients.
4. Conclusions
1. LH indicators in all the patients with HA were significantly higher than those in the control group, while FSH indicators in both groups were normal, but the ratio of the LH/FSH index was 3.04±0.58 in the first group, in the second group it was 2.19 ± 0.89 (p=0.07).2. AMH indicators are almost 2.5 times higher in group I than those in group II and average 9.2± 0.26 ng/ml and 3.7 ±0.2 ng/ml, respectively. A direct relationship of the AMH level with an increase in the level of androgens in the blood, an increase in the volume of the ovaries and the number of follicles in them has been established, which made it possible to develop a method for the early diagnosis of PCOS in hyperandrogenism syndrome.3. At an AMH level of more than 8.2 ng/ml, in combination with the presence of echographic signs of multifollicular ovaries, it can be regarded as the formation of polycystic ovary syndrome.4. In group I, the median of inhibin B - was 2 times higher than that in the control group (p=0.0009) and in group II it was 1.4 times (p=0.004). An increase in the level of inhibin B in patients with ovarian dysfunction, clearly correlated with both the level of AMH and the size of the follicles. In addition, in group I there was a significant direct correlation of inhibin B with the level of LH and the ratio of LH/FSH, which proved the LH-dependent nature of the increase in inhibin B in this category of patients.
References
| [1] | Unanyan, A.L. Syndrome of hyperandrogenism in the practice of a gynecologist / A.L. Unanyan, O.D. Rudnev. – M.: Status Praesens, 2014. – p.20. |
| [2] | Bogatyreva, E.M. Hyperandrogenism of puberty as a factor in reducing fertility / E.M. Bogatyreva, G.A. Novik, G.F. Kutusheva // Bulletin of Siberian Medicine. - 2016. - V. 15b No. 1. - pp. 14-21. |
| [3] | Azziz R., Carmina E., Chen Z., Dunaif A., et al. Polycystic ovary syndrome // Nat Rev Dis Primers. — 2016; 2: 16057. |
| [4] | Torgac M, Kokcu A, Cetinkaya MB, Alper T, Malatyalioglu E. Do basal inhibin A and inhibin B levels have value in the diagnosis of polycystic ovary syndrome? Gynecological Endocrinology 2005: 20: 322-326. |
| [5] | Hauzman EE, Fancsovits P, Murber A, Rabe T, Strowitzki T, Papp Z, Urbancsek J. Luteal-phase inhibin A and follicularphase inhibin B levels are not characteristic of patients with an elevated LH-to-FSH ratio. J Assist Reprod Genet 2006; 23: 141-147. |
| [6] | Fauser B.C., Tarlatzis B.C., Rebar R.W, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. FertilSteril. 2012; 97 (Suppl 1): 28.e25–38.e25. |
| [7] | Speiser P.W., Arlt W, Auchus R.J., Baskin L.S., Conway G.S., Merke D.P., et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an endocrine society clinical practice guideline. J ClinEndocrinolMetab. (2018) 103:4043–88. doi: 10.1210/jc.2018-01865. |
| [8] | Voutilainen R, Jääskeläinen J. Premature adrenarche: etiology, clinical findings, and consequences. J Steroid BiochemMol Biol. (2015) 145: 226–36. doi: 10.1016/j.jsbmb.2014.06.004. |
| [9] | Yang, R., Yang, S., Li, R., Liu, P., Qiao, J., Zhang, Y., 2016. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reproductive Biology and Endocrinology 14(1): 67. |
| [10] | Kostrzewa, M.; Głowacka, E.; Stetkiewicz, T.; Grzesiak, M.; Szyłło, K.; Stachowiak, G.; Wilczynski, J. Is serum anti-Müllerian hormone (AMH) assay a satisfactory measure for ovarian reserve estimation? A comparison of serum and peritoneal fluid AMH levels. Adv. Clin. Exp. Med.-Wroc. Med Univ. 2020, 29, pp.853–856. |
| [11] | Dumont, A.; Robin, G.; Dewailly, D. Anti-müllerian hormone in the pathophysiology and diagnosis of polycystic ovarian syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, pp.377–384. |
| [12] | Azizova G. D., Asatova M.M., Nadyrkhanova N. S., Dauletova M. J. Polycystic Ovary Syndrome As A Predictor Of Metabolic Syndrome In Women Of Reproductive Age Nat. Volatiles & Essent. Oils, 2021; 8(4): 15615-15618. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

