Sh. M. Muminov , D. L. Kim , B. P. Khamidov , T. A. Vervekina , D. D. Alimukhamedov
Republican Research Center of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The aim of the study. Development of a biocompatible biodegradable cava clip for the prevention of pulmonary embolism in patients with thrombosis of the iliocaval segment, which will not only create an obstacle for thromboembolism with preserved blood flow, but also after the disappearance of the risk of pulmonary embolism, be resorbed in a given time frame. Materials and Methods: The fabrication of the developed Biodegradable Cava Clips was carried out by preliminary 3D modeling and then printing them on a 3D printer. The experimental study was carried out in the conditions of the department of Experimental Surgery of the RRCEM. In total, the following laboratory animals were included in the study: 49 rats (25 white rats of the Vistar breed and 24 outbred rats), 6 outbred mice and 3 guinea pigs. The purpose of the experiment was to conduct a morphological study of the caudal vein and surrounding tissues after implantation of a biodegradable clip, determine the timing of resorption, and evaluate the toxicological safety indicators. Conclusions: according to the results of the study, a model of “biodegradable cava clip” was made from PGLA, which meets the requirements of toxicological safety. Morphological studies showed that on the 30th day of the experiment there were signs of degradation of the clip, and already on the 60th day there was a complete resorption of the clip. Microscopy revealed that the inflammatory infiltrate occupies 1-2 layers of the vessel wall, and its predominantly focal location is noted in the adjacent tissue. The replacement of the inflammation zone with connective tissue in the vessel wall and adjacent cellular tissue reaches a maximum by day 60, no gross changes in the endothelium and parietal thrombosis were detected.
Keywords:
Venous thrombosis, Iliocaval segment, Inferior vena cava, Pulmonary embolism, Biodegradable cava clips
Cite this paper: Sh. M. Muminov , D. L. Kim , B. P. Khamidov , T. A. Vervekina , D. D. Alimukhamedov , Development of a Biodegradable Cava Clips for the Thromboembolic Complications Prevention in Iliocaval Segment Thrombosis in the Experiment, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 447-455. doi: 10.5923/j.ajmms.20231304.19.
1. Introduction
Currently, according to various sources, deep vein thrombosis of the lower extremities (DVT) in the general population is recorded annually within 1-1.5 cases per 1000 adults, and pulmonary embolism (PE) is observed up to 60 cases per 100,000, and in the elderly and senile age, this figure rises to 200 cases per 100,000 population per year [1,2].Deep vein thrombosis of the IVC system is the main source of pulmonary embolism (84.5%). Given the magnitude of possible emboli from the IVC system, there is a high probability of massive thromboembolism of the pulmonary artery trunk or its branches with the development of acute respiratory failure and death [1,3,4]. Of course, the massiveness of the embolism depends on the diameter of the affected veins, and therefore the greatest danger occurs with thrombosis of the iliac-femoral segment, which, according to some authors, ranges from 5 to 15% [5,6,7].With regard to pulmonary embolism itself, for many years it has retained its third place due to the death of the population from cardiovascular diseases after myocardial infarction and stroke. Around the world, about 0.1% of the population dies from PE every year [2,8]. If we single out the number of people whose lethality is due to pulmonary embolism developing against the background of deep vein thrombosis, then one person out of every thousand is registered annually [9,10].It should be noted that today, despite the constant improvement of conservative therapy, in clinical practice there are patients with embolism-prone forms of thrombosis, when it is necessary to resort to methods of surgical prevention of pulmonary embolism. Currently, endo- and extravenous devices are known to create “metered” obstructions in the IVC and traps for large emboli.The cava filter belongs to endovenous devices, the advantage of which is considered by most authors to be low invasiveness. However, over time, observations have accumulated about the complications of this technique, both in the early and in the long-term period after the installation of the cava filter. The literature describes single attempts to create biodegradable cava filters from polyglycolic acid in the experiment [11,12]. However, these studies, the authors report, had several limitations, such as the lack of information about the exact time of degradation of materials in the bloodstream, methods for dynamically tracking filter changes, and the need for additional changes in filter designs to address the problem of filter migration. All this remains the main direction of future research.In parallel and alternatively to the endovascular method, the creation of extravasal devices was developed, the installation of which is carried out by an open surgical method. This method of surgical prevention of PE includes clipping of the inferior vena cava with a special cava clip, the principle of which is to divide the lumen of the vein into uniform collectors. Methods of cava clipping, in various modifications, were widely tested by Gordeev N.A., which were described in his dissertation for the degree of Doctor of Medical Sciences [13,14]. The clip was made of titanium or silver wire and had a number of technical advantages, and above all accessibility. Experiments have confirmed that the clip ensures the preservation of volumetric blood flow and there are no pathological changes in the tissues in the area of clipping. The positive aspects of this operation were also the possibility of using it in any surgical hospital, the absence of endothelial trauma, which is especially important in patients with a septic condition, the possibility of performing preliminary thrombectomy followed by the application of a cava clip. However, there is a significant drawback of this method - it is the preservation of a foreign body and partial narrowing of the lumen of the vein, as well as an increased risk of recurrence of deep vein thrombosis. The solution to this issue may be the use of biodegradable extravasal devices.
2. The Aim of the Study
Development of a biocompatible biodegradable cava clip for the prevention of pulmonary embolism in patients with thrombosis of the iliocaval segment, which will not only create an obstacle for thromboembolism with preserved blood flow, but also after the disappearance of the risk of pulmonary embolism, be resorbed in a given time frame.
3. Materials and Methods
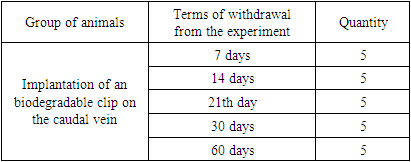
A. Choice of biocompatible biodegradable material. As a result of the search for the necessary biocompatible biodegradable material for the manufacture of the " Biodegradable Cava Clip", polyglycolide lactide (PGLA - a synthetic biodegradable surgical suture material consisting of copolymers of glycolide - 90% and L-lactide - 10%) was selected, which has excellent elasticity and provides high strength, does not have antigenic and pyrogenic properties and, when resorbed, cause a mild tissue reaction. The gradual decrease in tensile strength and the final resorption of PGLA threads occurs by hydrolysis at the implantation site, during which the polymer decomposes into glycolic and lactic acid. Absorption begins with a loss in tensile strength followed by a loss in mass. 80% of the original strength is retained up to 14 days from the date of implantation. 25% of the mass is absorbed in 4 weeks, and completely resorption is almost completed between 56 - 70 days.B. Experimental animals. In total, the following laboratory animals were included in the study: 49 rats (25 white rats of the Vistar breed and 24 outbred rats), 6 outbred mice and 3 guinea pigs.For the purpose of a morphological study during the implantation of the "Biodegradable cava clip", as well as to determine the timing of the resorption of the manufactured clip, 25 laboratory animals were included in the study - male and female white rats of the "Vistar" breed aged from 1 year to 1.3 years, weighing 200 -230 grams. The animals were divided into 5 groups depending on the timing of withdrawal from the experiment for further morphological studies.Table 1
 |
| |
|
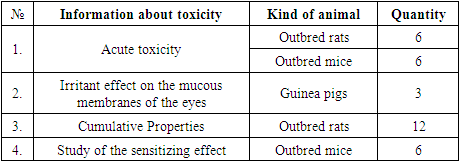
Evaluation of the toxicological safety parameters of "Biodegradable cava clip" was carried out on 24 outbred rats, 6 outbred mice and 3 guinea pigs. All animals were divided into groups depending on the methods of toxic exposure.Table 2
 |
| |
|
С. Method of making clips. 3D printing was chosen as the method for manufacturing experimental clips. The principle of 3D printing using any existing technology is the creation of three-dimensional objects from a set of flat layers. The digital model of the product is divided into layers by a special program - a slicer, and the printer prints these layers, one on top of the other, making up a three-dimensional object from them. So, from many layers, a three-dimensional detail is obtained.The manufacture of the developed "Biodegradable Cava Clips" was carried out by preliminary 3D modeling in the computer program Autodesk 3DsMax and Autodesk Maya, and then printing them on a 3D printer Picaso Designer X Pro (2018).Ethylene oxide (gas) sterilization was chosen as the sterilization method. Microbiological tests for sterility were carried out in a thioglycol medium at a temperature of 370C and in a Sabur medium at a temperature of 220C.D. Method of conducting experimental researchAll animals were operated on in the conditions of the Department of Experimental Surgery of the RRCEM. The operations were carried out using the operating magnifier Carl Zeiss "EyeMag Smart" 2.5x/450. After preliminary preparation and in accordance with the requirements of humane treatment, the experimental animals under general anesthesia with Isoflurane vapor underwent median laparotomy and implantation of prefabricated biodegradable PGLA clips for the experiment on the caudal vein.E. Method of morphological study of "Biodegradable cava clip".For microscopic examination, pieces of tissue were cut out from the area of the superimposed clip. After fixing the tissue in 10% neutral formalin in phosphate buffer (pH 7.2–7.4), the material was passed through alcohols of increasing concentration and embedded in paraffin according to the method of Z. Loyd et al. (1982). After that, serial sections with a thickness of 4-5 μm were made on an Accu-cut SRM rotary microtome (Sakura, Japan). The resulting sections were stained with hematoxylin and eosin using a Tssiue-Tek Plus apparatus (Sakura, Japan). The study of micropreparations to determine the qualitative changes in the microstructure was carried out on an Axioskop 40 microscope (ZEISS, Germany) with image fixation on a ProgRes CT3 camera.А. Method of toxicological evaluation of the safety parameters of the "Biodegradable cava clip".Biochemical blood tests were performed on a CYANSmart semi-automatic biochemical analyzer with software (Cypress Diagnostics, Belgium) according to standard methods (AST, ALT, ALP, total protein - Cypress Diagnostics reagent kits, Belgium), hematocrit was determined on a hematocrit centrifuge (Cypress Diagnostics , Belgium), a detailed analysis of peripheral blood was determined in the Goryaev chamber.Test design: for testing, an aqueous extract was prepared in distilled water from a kava clip in a volume ratio of 1:2, keeping for 24 hours in a thermostat at a temperature of 370C.To assess the effect on mucous membranes, the extract was injected into the conjunctival sac of guinea pigs (right eye - experience, left eye - control).The sensitizing effect was evaluated by an open application method. The extract was dosed at 0.05 cm3 per gauze swabs placed on polyethylene pads, which were fixed on the window, and the animal was placed in a restrictive movement machine. Distilled water was similarly applied to the control skin "window" in the same amount.During the experiment, changes in the general condition, motor activity, condition of the coat and skin, as well as their behavioral reactions, were recorded.
4. Results
A. Results of the manufacture of biodegradable clips for an experimental study on laboratory animals.As a result of the selected biocompatible biodegradable material (PGLA) and the method of making cava clips by printing on a 3D printer at a melting temperature of 2300C, coils with wound PGLA threads with a diameter of 1.75 mm and a length of 77 meters were purchased (Fig. 1).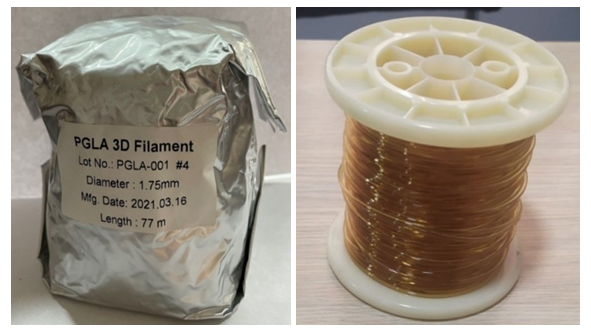 | Figure 1. PGLA filament spool for 3D printing |
The first stage for experimental studies on laboratory animals (rats) was to preliminarily fabricate small V-shaped clips on a 3D printer (Fig. 2), taking into account the anatomical features of laboratory animals (the diameter of the caudal vein of rats is less than 1.5 mm).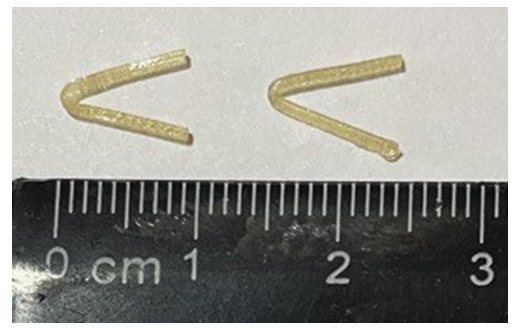 | Figure 2. V-shaped PGLA clips |
Subsequently, after manufacturing, ethylene oxide (gas) sterilization of the clips was carried out. Microbiological tests for sterility were carried out in a thioglycol medium at a temperature of 370C and Sabur's medium at a temperature of 220C. According to the results of tests in both environments, the test material after the above sterilization method corresponded to the parameter - sterile. Next, the clips were placed in a sterile package (Fig. 3). | Figure 3. Sterile packaging |
B. Results of morphological examination after insertion of biodegradable clips in laboratory animals.Experimental animals under general anesthesia with Isoflurane pairs underwent median laparotomy up to 3 cm long. After wrapping the edges of the wound with sterile napkins, an expander was installed early, intestinal loops were removed from the abdominal cavity and placed to the right of the surgical wound. The inferior vena cava below the level of the confluence of the renal veins was exposed by sharp and blunt means (Fig. 4).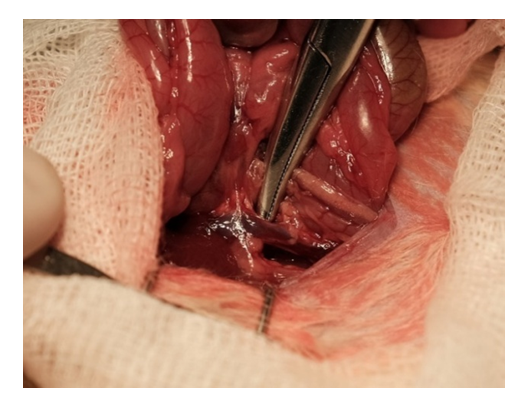 | Figure 4. Isolation of the inferior vena cava |
After the vein was freed from adhesions within the 2-5 mm segment, a clip of the appropriate size, made on a 3D printer from biodegradable PGLA material, was installed. The length of the clip branches is up to 8 mm, the opening angle is up to 300. After installing the clip, its ends approached until the vein lumen was partially closed (Fig. 5). The necessary hooking of the ends of the clip was provided by fixing the approximate ends of the jaws into the thickness of the tissues. The surgical wound was sutured.macroscopic picture.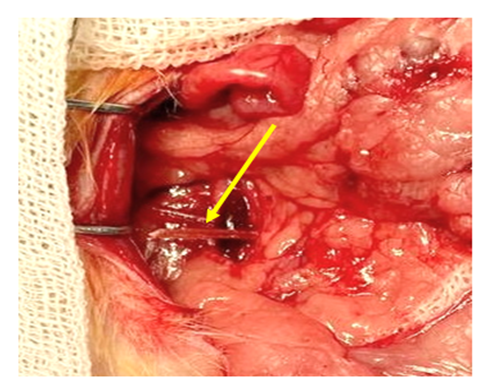 | Figure 5. The moment of insertion of the clip made of biodegradable PGLA material (the arrows indicate the clip) |
Animals were withdrawn from the experiment in groups on days 7, 14, 21, 30 and 60 after surgery by isoflurane overdose, with an assessment of the macro- and microscopic response of tissues to the installed implant.Macroscopic examination in all 5 groups (Fig. 6) after opening the abdominal cavity in animals did not reveal the presence of free fluid. In the abdominal cavity, no pronounced adhesive process was noted. A moderate adhesive process was noted in the retroperitoneal space. After mobilization of the site of application of the biodegradable clip, it was found that there was no dislocation of the clip in groups I-IV, the clip retained its predetermined shape at these times, and there was no hematoma around the clip. Signs of vein thrombosis, periphlebitis, as well as bedsores and wall perforations were not observed at all times. On the 30th day of the experiment, there were signs of degradation of the clip, and already in group V, complete resorption of the clip was noted.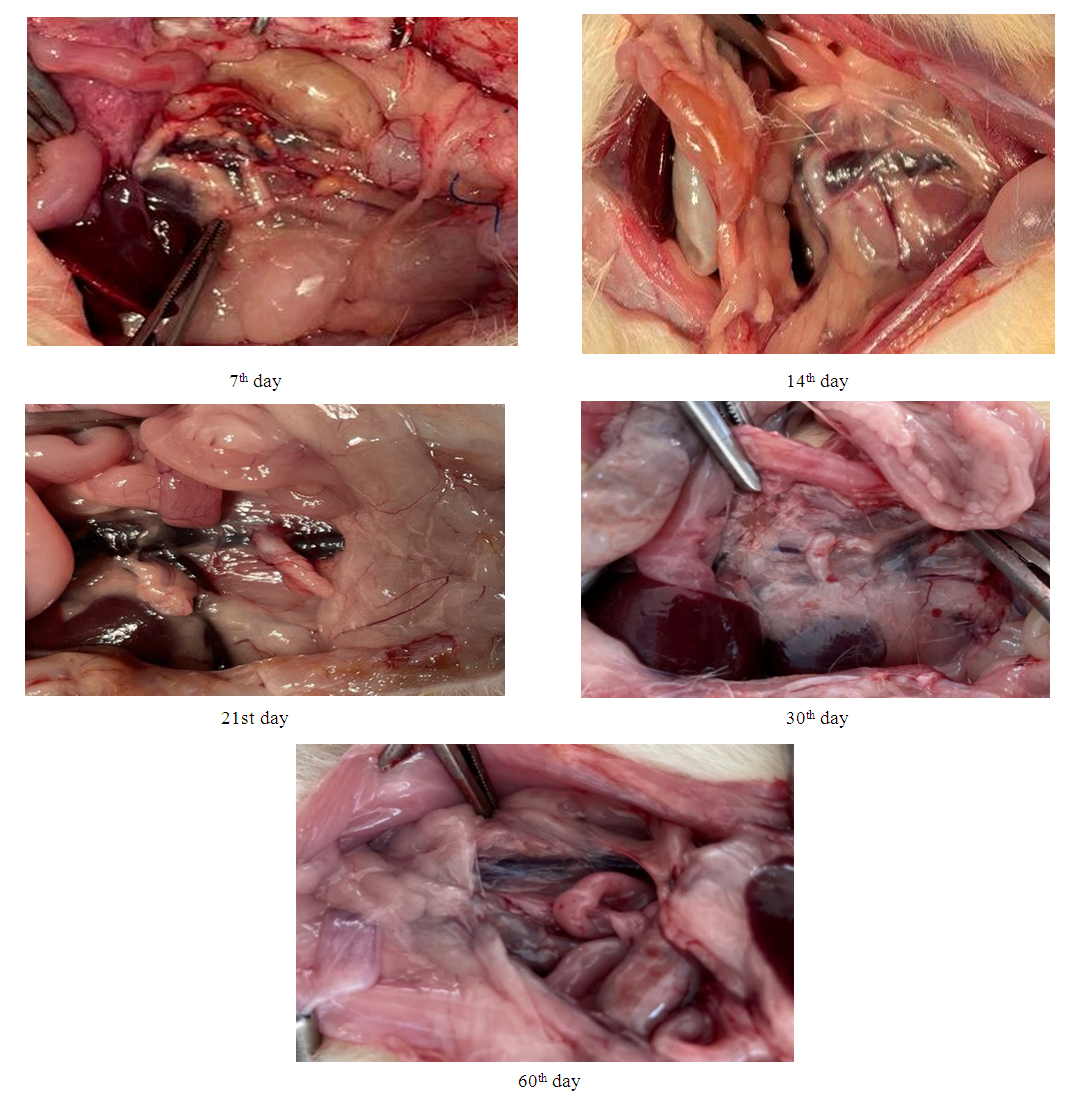 | Figure 6. Macroscopic picture after withdrawal from the experiment at different times |
Microscopic picture.Group I (7th day after applying the clip).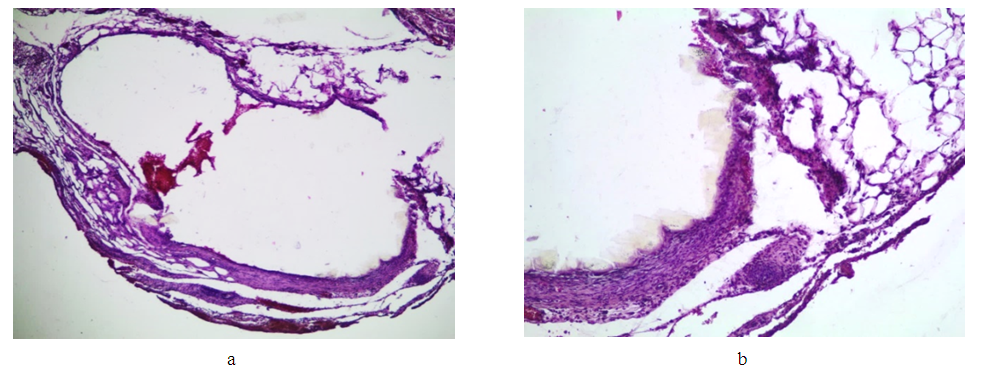 | Figure 7. Changes in the IVC wall on the 7th day after clip installation. Staining with hematoxylin and eosin: a) deformation of the lumen of the vein (lens magnification 4, eyepiece 10); b) inflammatory infiltration of the wall and adjacent adipose tissue (lens magnification 4, eyepiece 10) |
Group II (14th day after applying the clip).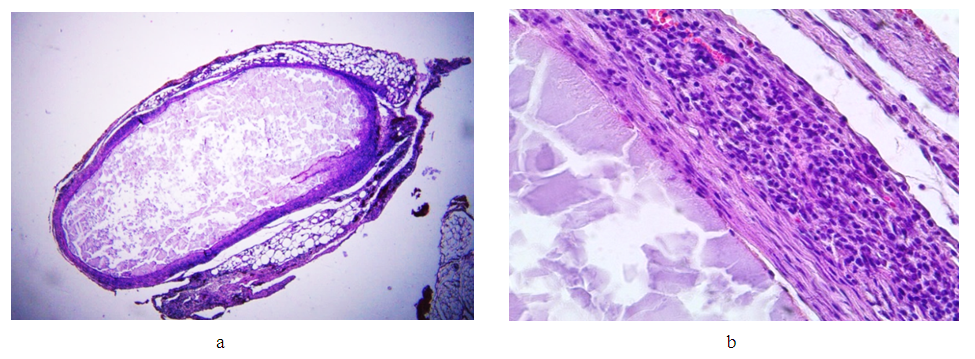 | Figure 8. Changes in the wall of the IVC on the 14th day. Stained with hematoxylin and eosin. a) bean-shaped deformation of the lumen (lens magnification 1.25, eyepiece 10); d) inflammatory infiltration of adventitia and media (lens magnification 40, eyepiece 10) |
Conclusion: a comparison of the changes detected on days 7 and 14 after the installation of the clip on the venous trunk clearly demonstrates the increase in pathological processes in the second group (day 14), with an increase in the depth of wall invagination into the lumen of the vessel, its deformation, an increase in the degree of inflammation of the venous trunk and tissues adjacent to it. Attention is drawn to the spread of the inflammatory infiltrate of the vein wall from the adventitious layer to the endothelium, with its minimal damage. At the same time, with an increase in time after surgery, the volume of granulation tissue increases in the areas adjacent to the vessel, as well as in the deformed areas of the vein wall.Group III (21st day after applying the clip).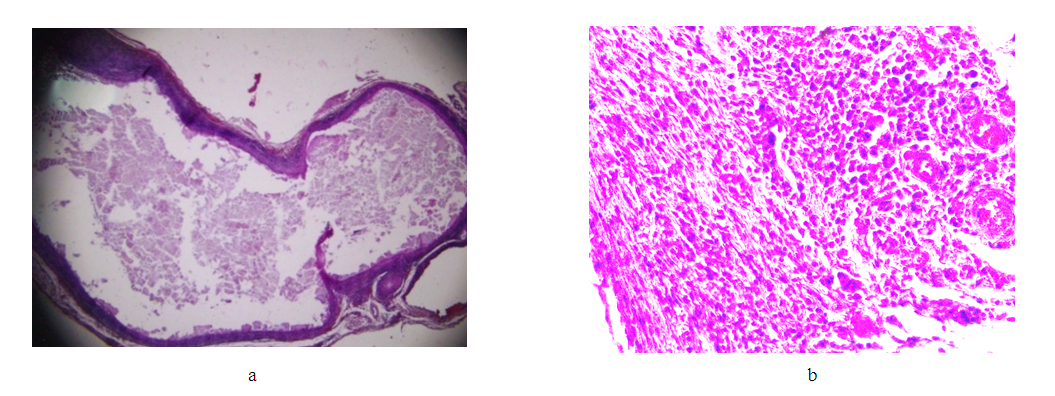 | Figure 9. Changes in the wall of the IVC on the 21st day. Stained with hematoxylin and eosin. a) bean-shaped deformation of the lumen (lens magnification 1.25, eyepiece 10); b) immature granulation tissue in the vessel adventitia (objective magnification 40, eyepiece 10) |
Result. Comparison of the changes identified on days 7, 14 and 21 after the installation of the clip on the venous trunk clearly demonstrates the increase in pathological processes in the second group (day 14), with an increase in the depth of wall invagination into the lumen of the vessel, its deformation, an increase in the degree of inflammation of the venous trunk and tissues adjacent to it. This process progresses in the third group (21 days). Attention is drawn to the spread of the inflammatory infiltrate of the vein wall from the adventitious layer to the endothelium, with its minimal damage. However, with an increase in the exposure time of the clip (21 days), the intensity and area of cellular infiltration decreases, with the formation of predominantly lymphoid infiltrates both in the vessel wall and in the adjacent adipose tissue. At the same time, with an increase in time after surgery, the volume of granulation tissue increases in the areas adjacent to the vessel, as well as in the deformed areas of the vein wall.Group IV (30th day after applying the clip).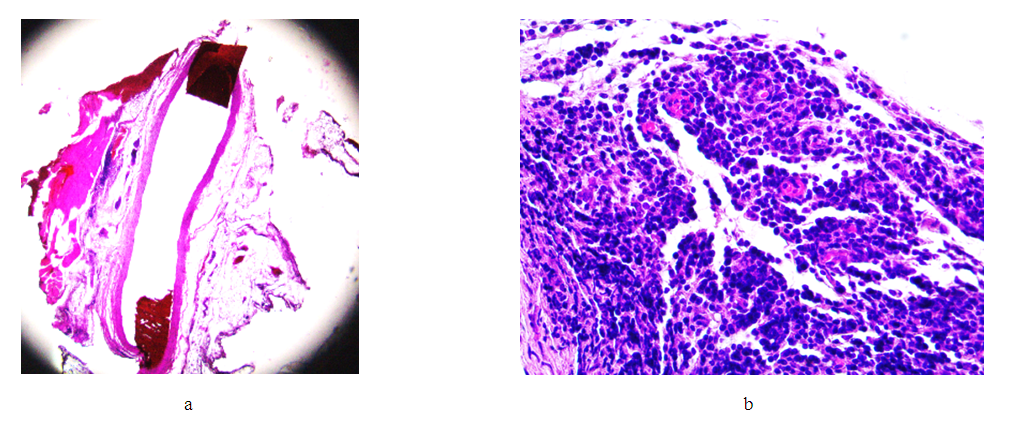 | Figure 10. Changes in the wall of the IVC on the 30th day. Stained with hematoxylin and eosin. a) bean-shaped deformation of the lumen (lens magnification 1.25, eyepiece 10); b) granulation tissue in the vessel adventitia and adjacent tissue (objective magnification 40, eyepiece 10) |
Conclusion. Comparison of the changes identified on days 7, 14, 21 and 30 after the installation of the clip on the venous trunk clearly demonstrates the increase in pathological processes in the second group (day 14), with an increase in the depth of wall invagination into the lumen of the vessel, its deformation, and an increase in the degree of inflammatory damage to the venous trunk and adjacent tissues. This process progresses in the third group (21 days). Attention is drawn to the spread of the inflammatory infiltrate of the vein wall from the adventitious layer to the endothelium, with its minimal damage. However, with an increase in the exposure time of the clip (21 days), the intensity and area of cellular infiltration decreases, with the formation of predominantly lymphoid infiltrates both in the vessel wall and in the adjacent adipose tissue. At the same time, with an increase in time after surgery, the volume of granulation tissue increases in the areas adjacent to the vessel, as well as in the deformed areas of the vein wall. But already by the 30th day, the granulation tissue begins to be replaced by loose fibrous tissue, foci of fibrous tissue both in the vessel wall and in the adjacent tissue. Damage to the endothelium and thrombotic masses in the lumen of the vessel in this group are absent.Group V (60th day after applying the clip).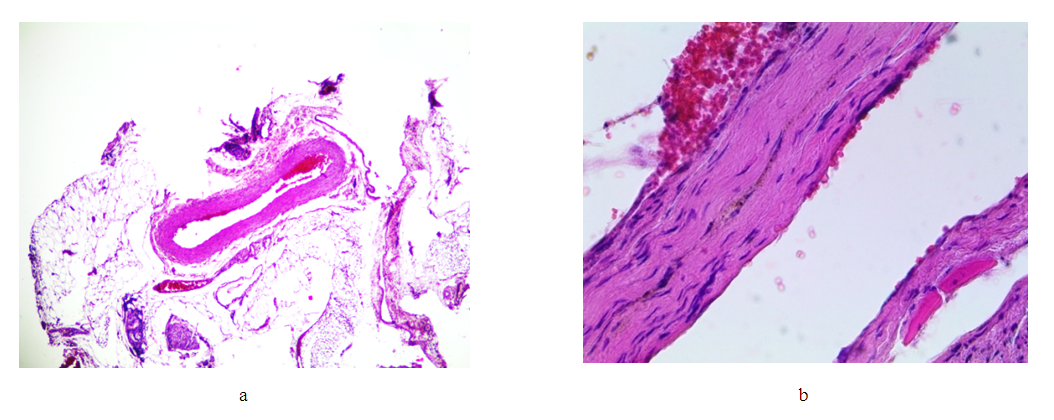 | Figure 11. Changes in the wall of the IVC on the 60th day. Stained with hematoxylin and eosin. a) bean-shaped deformation of the lumen (lens magnification 4, eyepiece 10); b) replacement of the wall layers with connective tissue while maintaining the structure of the endothelium (lens magnification 40, eyepiece 10) |
Result. Comparison of the changes identified in the five study groups - 7, 14, 21, 30 and 60 days after the installation of the clip on the venous trunk, clearly demonstrates the increase in inflammatory processes in the first three groups. At the same time, the peculiarity of the distribution of the inflammatory infiltrate is quite interesting. If in the first 7-14 days the infiltrate spreads in almost all layers of the wall, and diffusely in the adjacent tissue, then by 21-30 days the infiltrate occupies 1-2 layers of the vessel wall, and its predominantly focal location is noted in the adjacent tissue. The replacement of the inflammation zone with connective tissue in the vessel wall and adjacent cellular tissue reaches a maximum by 60 days. The deformation of the vessel reaches a maximum by 30 days and practically does not change up to 60 days. In all study groups, there were no gross changes in the endothelium and parietal thrombosis.B. Design of the "Biodegradable Cava Clip" model.The prototype of the “Biodegradable Cava Clip” was the titanium “Cava Clip” utility model according to patent No. FAP 01857 publ. 04/30/2022 in Bull. No. 4. The disadvantage of this solution was that the cava clip is made of titanium wire, and after installation, the clip remains in the body on a permanent basis in the form of a foreign body, also permanently maintaining a partial narrowing of the inferior vena cava and increasing the risk of recurrent deep vein thrombosis.The “biodegradable cava clip” is a single clip of two elastically connected jaws, made of PGLA by preliminary 3D modeling and printing on a 3D printer at a melting temperature of 230°C (Fig. 12).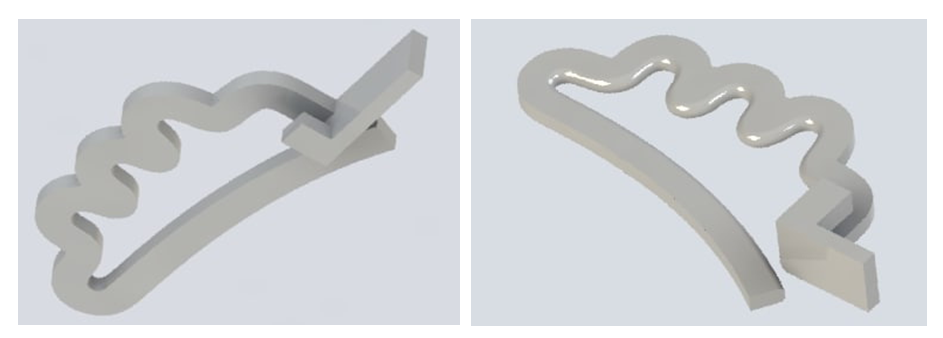 | Figure 12. 3D clip modeling |
The upper branch has a wave-like curved section with a U-shaped hook at one end. The lower jaw of the device is arc-shaped, repeating the curve of the anterior surface of the body of the lumbar vertebra, the end of which is designed to engage with the end portion of the upper jaw to close the clip.As a result of this decision, the possibility of biodegradation of the cava clip was achieved not earlier than 22 days after its installation, i.e. after the disappearance of the risk of thromboembolic complications in order to restore the lumen of the vein, rid the body of a foreign body and reduce the risk of recurrence of deep vein thrombosis.After 3D modeling, the final model of the biodegradable cava clip is produced from biodegradable PGLA material by 3D printing at a melting temperature of 230°C (Fig. 13).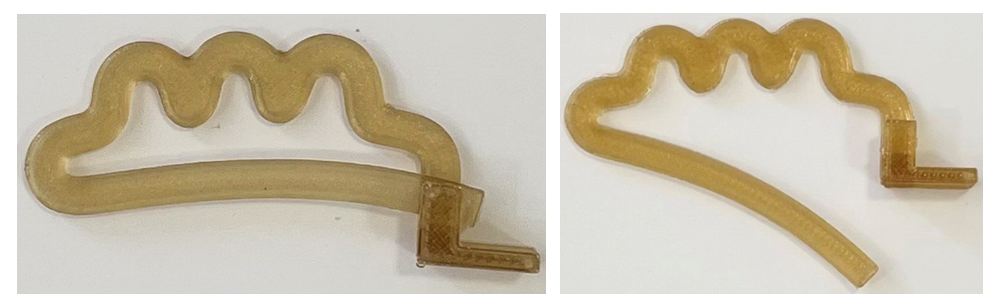 | Figure 13. PGLA biodegradable cava clips |
The device is used as follows: if there are indications for surgical prevention of pulmonary embolism in case of thrombosis of the veins of the lower extremities and pelvis, an operation is performed - cava clipping. Operative access (modified according to Rob, laparotomy or pararectal) depends on the volume of the planned operation. Through the retroperitoneal space on the right, the inferior vena cava is isolated and mobilized in a small area (4.0 - 5.0 cm) at the level of LII - LIV in the infrarenal region. The cava clip, opened from two elastically connected branches, is passed from the side of the abdominal aorta with its lower arcuate branch under the inferior vena cava, and the upper branch, which has a wave-like curved section, over the inferior vena cava. Next, the lower and upper branches are brought together and the end of the lower branch is closed with the U-shaped hook of the upper branch.After closing the wavy section of the upper branch with the lower arcuate branch of the clip, the lumen of the vein is divided into equal intervals (collectors), the diameter of which is three to four times less than the lumen of the IVC.Thus, protection is provided from the passage of thromboembolism through the clipped section of the inferior vena cava to the heart from the deep veins of the lower extremities and pelvis and blood flow is maintained above and below the device without creating a pressure gradient.Subsequently, prototypes of the final model of the “Biodegradable Cava Clip” were subjected to physical and mechanical tests, according to the results of which the “Breaking load” was 10 N, which is sufficient for the IVC, given the absence of pulsating blood flow in it.The finished final model of the “Biodegradable Cava Clip” is patented by the Agency for Intellectual Property under the Ministry of Justice of the Republic of Uzbekistan.D. The results of the evaluation of the toxicological safety parameters of the "Biodegradable cava clip".According to the results of the study, no lethal outcome was observed among the experimental animals during the period of the experiment. There were also no clinical signs of intoxication during the experiment. Observations of changes in the body weight of rats showed that throughout the experiment, the animals of the experimental groups gained weight and the degree of weight gain in the experimental animals did not significantly differ from the weight of the animals in the control group.Result. The conducted tests showed that the aqueous extract into distilled water from the " Biodegradable cava clip" does not have a systemic toxic effect, does not cause irritation of the mucous membranes of the eye (Iir - 0 points) and does not have a sensitizing effect (Is - 0 points). Therefore, the "Biodegradable Cava Clip" made of biodegradable medical material PGLA meets the safety requirements.
5. Conclusions
1. As a result of studying and searching for the necessary biocompatible biodegradable material for the manufacture of the “Biodegradable cava clip”, polyglycolide lactide (PGLA is a synthetic biodegradable surgical suture material consisting of copolymers of glycolide - 90% and L-lactide - 10%) was selected. The gradual decrease in tensile strength and the final resorption of PGLA threads occurs by hydrolysis at the implantation site, during which the polymer decomposes into glycolic and lactic acid. Absorption begins with a loss in tensile strength followed by a loss in mass. 80% of the original strength is retained up to 14 days from the date of implantation. 25% of the mass is absorbed in 4 weeks, and completely resorption is almost completed between 56 and 70 days.2. A model of a cava clip made of biodegradable material and a method for its manufacture by preliminary 3D modeling and printing on a 3D printer have been developed. Ethylene oxide (gas) sterilization was chosen as the clip sterilization method, after which the test material corresponded to the sterile parameter. According to the results of physical and mechanical tests, the breaking load was 10 N, which is sufficient for the IVC, given the absence of pulsating blood flow in it.3. Conducted tests to assess the toxicological safety indicators of the “Biodegradable Cava Clip” showed that the clip made of biodegradable medical material PGLA complies with safety requirements.4. Macroscopic examination in all 5 groups of internal organs showed no macroscopic changes. There was no dislocation of the clip in groups I-IV, the clip retained its predetermined shape at these times, there were no hematomas around the clip, signs of vein thrombosis, periphlebitis, as well as bedsores and perforation of the walls were not observed. On the 30th day of the experiment, there were signs of degradation of the clip, and already on the 60th day, complete resorption of the clip was noted. When comparing the changes during microscopic examination, identified in five study groups - 7, 14, 21, 30 and 60 days after the installation of the clip on the venous trunk, clearly demonstrates the increase in inflammatory processes in the first three groups. At the same time, the peculiarity of the distribution of the inflammatory infiltrate is quite interesting. If in the first 7-14 days the infiltrate spreads in almost all layers of the wall, and diffusely in the adjacent tissue, then by 21-30 days the infiltrate occupies 1-2 layers of the vessel wall, and its predominantly focal location is noted in the adjacent tissue. The replacement of the inflammation zone with connective tissue in the vessel wall and adjacent cellular tissue reaches a maximum by 60 days. The deformation of the vessel reaches a maximum by 30 days and practically does not change up to 60 days. In all study groups, there were no gross changes in the endothelium and parietal thrombosis.
References
| [1] | Tsibulkin N.A., Frolova E.B., Abdrakhmanova A.I., Tukhvatullina G.V. Modern problems of pathogenesis and diagnosis of pulmonary embolism. Practical Medicine 2020; 18(1): 8-12 (In Russ.) |
| [2] | Wendelboe A., McCumber M, Hylek EM, Buller H, Weitz JI, Raskob G. Global public awareness of venous thromboembolism. ISTH Steering Committee for World Thrombosis Day. J. Thromb Haemost. 2015; 13(8): 1365-1371 (Boston University School of Medicine, Boston, MA, USA). |
| [3] | Saveliev B.C., Yablokov E.G., Kirienko A.I. Massive pulmonary embolism. Medicine 1990: 336 p. (In Russ.) |
| [4] | Kline JA, Kabrhel C. Emergency Evaluation for Pulmonary Embolism, Part 1: Clinical Factors that Increase Risk. J Emerge Med. 2015: 48(6): 771-780. doi: 10.1016/j.jemermed.2014.12.040. Epub 2015 Apr 8 (Harvard Medical School, Boston, Massachusetts). |
| [5] | Ignatiev I. M., Akchurin F. R., Zanochkin A. V. et al. Experience in the treatment of floating thrombosis in the system of the inferior vena cava. Phlebology 2011: 4: 44–51 (In Russ.) |
| [6] | Khadzhibaev A.M., Asamov R.E., Khamidov B.P., Isamukhamedov Sh.Sh. Diagnosis and choice of treatment tactics in patients with thrombosis of the inferior vena cava system. Problems of Clinical Medicine 2007: 1(9): 67-73. |
| [7] | Shulutko A. M., Krylov A. Yu., Osmanov E. G. Anticoagulant therapy in the treatment of acute proximal deep vein thrombosis of the lower extremities. Surgery 2011: 1: 52–55. (In Russ.) |
| [8] | Heit J.A. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol., 2015; 12: 464–474. |
| [9] | Nikulina N. N., Terekhovskaya Yu. V. Epidemiology of pulmonary embolism in the modern world: analysis of morbidity, mortality and problems of their study. Russian journal of cardiology. 2019; 24(6): 103–108. |
| [10] | Dorohina K.R., Khromtsova O.M., Fominykh M.I. The prevalence of pulmonary embolism in different countries of the world Medical Bulletin of Bashkortostan 2019: 14(6): 48-53 (In Russ.) |
| [11] | Fuxian Zhang, Hailei Li, Gangzhu Liang, Huan Zhang. Development and evaluation of a new biodegradable vena cava filter in a canine model. Asian Journal of Surgery 2017; 40(1): 12-16. |
| [12] | Glushchenko L.V., Sharafeev A.Z., Shchepochkin V.A. Self-dissolving kava filter. Company LLC IK Modern Technologies 2014. https://navigator.sk.ru/orn/1120757 (In Russ.) |
| [13] | Gordeev N.A. Surgical prevention of pulmonary thromboembolism arteries in iliac-femoral phlebothrombosis: dis. … Dr. med. Sciences: 14.00.44. Gordeev N.A. SPb. 1997; 256 p. (In Russ.) |
| [14] | Gordeev N.A., Sedov V.M., Ballyuzek F.V., Myasnikova M.O., Khon A.E., Yurlov V.V., Shabanova N.A. Prevention of pulmonary embolism by clipping the inferior vena cava and iliac veins: indications, technique, immediate and long-term results. Surgery News 2010; 18(4): 157-164 (In Russ.) |










 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





