-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(4): 440-446
doi:10.5923/j.ajmms.20231304.18
Received: Mar. 30, 2023; Accepted: Apr. 14, 2023; Published: Apr. 22, 2023

Locally Produced Aluminium-Free and Commercial Aluminium-Containing Antiperspirants Compared
Pharm. Favour I. Olusanya, Professor Ogbonna Okorie, Dr Onyenoha Chukwumerije, Pharm. Oluwatomi T. Olusanya
Faculty of Pharmaceutical Sciences University of Port Harcourt, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Pharm. Favour I. Olusanya, Faculty of Pharmaceutical Sciences University of Port Harcourt, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

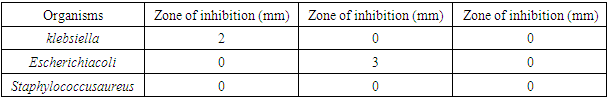
Objective: This study aimed at comparing the effectiveness of a locally formulated aluminium-free antiperspirant with a commercial aluminium-containing product. Materials and Methods: The flower buds of clove were obtained, identified as UPHM313 in the herbarium, washed, dried, powdered and extracted using acetone. The tannin component was further derived using soxhlet extraction technique. Tannins were identified using phytochemical tests followed by preparation of the antiperspirant using different concentrations of the extract; a placebo containing only the excipients was also formulated. Evaluation of the effectiveness of the antiperspirant produced, the placebo and a commercially available antiperspirant containing aluminum, was performed via antimicrobial susceptibility testing to the bacteria isolated from the armpits of female volunteers: Staphylococcus aureus, Pseudomonas, Escherichia coli, Klebsiella. Results: The crude tannin extract was shown to have antimicrobial effect. The result showed increased inhibitory effect with an increase in concentration. At 10.00% concentration of the crude extract in the formulation, the average zones of inhibition of Staphylococcus, Escherichiacoli and Klebsiella were 0mm, 4mm and 3.7mm, respectively. At 25.00% concentration, 16mm, 0mm and 27.7mm, respectively while the commercial antiperspirant had an inhibition of 0mm, 1mm and 0.7mm, respectively. The antiperspirant formulated with the crude extract of Syzygiumaromaticum had a wider inhibitory effect against the microorganisms present in the armpit of female volunteers compared to the antimicrobial effect of commercial brand of antiperspirant having aluminium as its active agent on the same microorganisms. Conclusion: The antiperspirant formulated with crude extract of clove was found superior to the commercial brand antiperspirant containing aluminium. Recommendation: Further similar study needs to be conducted with a larger sample size for more evidence-based recommendations.
Keywords: Comparison, Aluminium-containing antiperspirant, Aluminium-free antiperspirant, Port Harcourt, Nigeria
Cite this paper: Pharm. Favour I. Olusanya, Professor Ogbonna Okorie, Dr Onyenoha Chukwumerije, Pharm. Oluwatomi T. Olusanya, Locally Produced Aluminium-Free and Commercial Aluminium-Containing Antiperspirants Compared, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 440-446. doi: 10.5923/j.ajmms.20231304.18.
Article Outline
1. Introduction
- It is a current international discuss in the pharmaceutical literature of the role of alumimium salts present in most antiperspirants in the aetiology of cancer in humans [1-8]. In addition to the safety of the antiperspirant, a major requirement in any antiperspirant is its effectiveness during use. Some chemical antiperspirants are effective, however, the side effects like allergy and irritations are also obvious [5].The adverse effects caused by chemical antiperspirants could be avoided by using natural plant extracts. If use of underarm cosmetics is a factor in the development of breast cancer, then options for prevention could at last become a reality through individual decisions to cease usage or through alterations to product formulations [5]. In pursuant of the desire to popularise bystander CPR and possibly have its teaching and training incorporate Natural ingredients have been used for centuries for skin care purposes.Nowadays, they are becoming more prevalent in formulations, due to consumers’ concerns about synthetic ingredients/chemical substances. The main benefits reported for plant extracts, used in skin care, include antioxidant and antimicrobial activities and tyrosinase inhibition effect. In this review, some examples of plants from Portuguese flora, whose extracts have shown good properties for skin care are presented. However, despite the known properties of plant extracts, few studies reported the development of formulations with them. More work in this field can be accomplished to meet consumer demand [9]. The use of bioactive extracts or phytochemicals from a variety of botanicals in cosmetics accomplishes two functions: care of the body and as ingredients to influence the biological functions of the skin, providing the nutrients for healthy skin. Generally, botanical products are a rich source of vitamins, antioxidants, essential oils and oils, hydrocolloids, proteins, terpenoids and other bioactive compounds. According to their composition, these extracts can provide different properties including Antioxidant Activity [10].Meanwhile, there is increasing demand for antiperspirant globally including African countries, as well as in the United Arab Emirate [11-13]. In order to contribute in meeting this growing demand for antiperspirants, there is need for more research in this area so as to provide evidence-based solutions.In line with the link between sustainable development goals (SDGs) on health [14] and Agenda 2063: The Africa We Want [15] policies, this study aimed at comparing two different antiperspirants: a locally produced aluminium salts-free antiperspirant and an aluminium salts-containing commercial product. It was hypothesized that the locally produced aluminium salts-free product would not be as effective as the aluminium salts-containing commercial foreign-produced antiperspirant in handling the bacteria involved in armpit sweating in humans. (Cohorts). of participants who previously were trained separately using hands-only and conventional (standard) CPR methods. It was hypothesized that there would be no statistically significant differences in the retained CPR skills in the three common CPR skills domains of the two groups 15 months after their initial trainings.
2. Materials and Methods
- The detailed materials and methods used as earlier reported by Olusanya et al [16] is shown below: MaterialsClove flower buds, Cyclomethicone, Stearyl alcohol, Elaeis guineensis (palm) kernel oil, C12-13 alkyl benzoate, PPG-14 butyl ether, Hydrogenated castor oil, Hydrogenated soya bean oil.
2.1. Laboratory Devices Used
- Soxhlet extractor, Evaporating dish, Desiccator, Rotatory evaporator, Water bath, Evaporating dish.
2.2. Collection and Identification of Clove Flower Bud
- Syzgium aromaticum flower bud were obtained from the Mile3 market, Rivers State, Port Harcourt and identified by Dr. Suleiman Mikailu and deposited in the herbarium of Pharmacognosy and Phytotherapy Faculty of Pharmaceutical Sciences, University of Port Harcourt. It was identified as herbarium number UPHM313.
2.3. Preparation of Plant Extract
- The Syzgium aromaticum were washed, then dried. The dried material was ground until it became powdry and subsequently sieved The powdered material was stored in dried condition until it was extracted with Acetone and the acetone extract obtained.
2.4. Extraction of Tannins from Clove
- 50g of the powered flower bud of clove was placed into the soxhlet extractor at 56°C boiling point. The solvent was heated to reflux. The solvent vapour travelled up a distillation arm and flooded into the chamber housing the powered clove. The condenser ensured that the solvent vapour cools and drips back down into the chamber housing the powered clove. The chamber containing the powdered clove was slowly filled with warm acetone. The acetone solvent returns back to the distillation flask when the chamber is full. The cycle was repeated many time for 3 days until exhaustive extraction of tannins from clove was achieved.
2.5. Separation of Solvent from Extract
- This was done using a Rotarty Evaporator [17] in the Pharmaceutical Chemistry laboratory, Pharmaceutical Sciences, University of Port Harcourt.
2.6. Phytochemical Screening for Tannins
- Phytochemical Screening; It refers to the extraction, screening and identification of the medicinally active substance found in plants. Some of the bioactive substances that can be derived from plants are flavonoids, alkaloids, carotenoids, tannin, antioxidants and phenolic compounds [18].
2.6.1. Ferric Chloride Tests
- For the detection of tannins, firstly 5 ml of the extracts filtrate was boiled for 5 minutes along with 5ml solution of 45% ethanol. The mixture was then allowed to cool at room temperature ant was filtered using filter paper. 1 ml of the filtrate was then taken in a test tube and diluted with 2ml of distilled water. Few drops of ferric chloride were then added to the solution. A greenish to black color appeared, indicates the presence of tannins [18].
2.6.2. Other Phytochemical Screening
- Alkaloid precipitation, goldbeater’s skin test [18].
2.7. Preparation of Tannin Antiperspirant
- The emulsions were obtained using the required ingredients that have been weighed / measured according to their calculated values. The fat soluble ingredients for the different emulsions prepared are: Stearyl alcohol, Corn starch, Hydrogenated soya bean oil, Hydrogenated castor oil, C12-13 alkyl benzoate, Palm kernel oil and the water soluble ingredients are: Cyclomethicone, PPG-14 butyl ether and tannins.
2.7.1. Formula for the Preparation of the Antiperspirant with Crude Tannin Extract (10.00%)

2.7.2. Formula for Formulation of Antiperspirant Using 25.00% Crude Tannin Extract
 Mixing ProcedurePhase A was heated to 65°C. Phase B was added. it was mixed using an homogenizerPhase C was added to Phase A + B. mixing was continued for 30minsPhase D was added to Phase A + B +C and it was mixed to homogenized emulsion was achieved. It was cooled to 35°C [19].Mixing ProcedurePhase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizerPhase C was added to Phase A+B. missing was commenced for 30minutesPhase D was added to Phase A+B+C and it was mixed until homogenous. It was cooled to 35°C [19].
Mixing ProcedurePhase A was heated to 65°C. Phase B was added. it was mixed using an homogenizerPhase C was added to Phase A + B. mixing was continued for 30minsPhase D was added to Phase A + B +C and it was mixed to homogenized emulsion was achieved. It was cooled to 35°C [19].Mixing ProcedurePhase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizerPhase C was added to Phase A+B. missing was commenced for 30minutesPhase D was added to Phase A+B+C and it was mixed until homogenous. It was cooled to 35°C [19].2.7.3. Formula for Preparation of Antiperspirant without Crude Tannin Extract
 Mixing ProcedurePhase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizerPhase C was added to Phase A+B. missing was commenced for 30minutesPhase D was added to Phase A+B+C and it was mixed until homogenous. It was cooled to 35°C [19].
Mixing ProcedurePhase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizerPhase C was added to Phase A+B. missing was commenced for 30minutesPhase D was added to Phase A+B+C and it was mixed until homogenous. It was cooled to 35°C [19].2.8. Evaluation of Antiperspirant
- Microbial analysis of crude extract, antiperspirant product and placebo.
2.8.1. Collection of Bacterial Isolate
- Four female volunteers were directed not to apply any antiperspirant or deodorant directly to the underarm. After the day’s activity they were taken to the Pharmaceutical microbiology laboratory of Pharmaceutical Science University of Port Harcourt and sterile swabs were taken on the underarm of each volunteer. The swabs were streaked in the nutrient plates: Nutrient agar, Mac and MSA and incubated for 24hours for microbial growth at 36°C [20].
2.8.2. Microbial Identification
- Microorganisms can sometimes be identified on the basis of how often they appear on or in different media; the pigmentation, size, odour, haemolysis on blood and shape of microbial colonies as they appear on or in the different agar plates [21].
2.8.2.1. Gram Staining
- This is a differential technique used to identify bacteria based on their characteristics either as Gram positive or Gram negative organisms which is based on the ability of cell wall to retain a certain stain or not [21].ProcedureThe isolated organisms from the plates was smeared on a clean glass slide by placing a loopful of water and an inoculum on the slide and allowed to air dry. The smear was then heat fixed and allowed to dry. The prepared smear was flooded with to 1% crystal violet dye for 1 minute, after which the excess stain was rinsed off using distilled water, the smear was then flooded with Lugol’s iodine for I minute, the iodine was rinsed off with distilled water, after which the smear was also flooded with 98% alcohol for 30 seconds, rinsed quickly with distilled water and then Safranin red was added. Allowed for 1 minute and rinsed off with distilled water. The various smears were air dried for few minutes and a drop of oil immersion was added and observed under the light microscope. The same procedure was repeated for the different test results [21].
2.8.2.2. Biochemical Test
- The biochemical tests were carried out on the test isolates obtained. They include the following:Catalase TestThis test is used to differentiate bacteria that produce catalase enzyme from those that does not produce the enzyme. The function of the enzyme is to detoxify hydrogen peroxide formed from the superoxide dismutase by breaking it down into water and oxygen gas2H2O2 CATALASE 2H2O + O2ProcedureA drop of 3% hydrogen peroxide was placed on a clean non greasy microscope slide. A flamed and cooled wire loop was used to emulsify a colony of each isolated microorganism on the drop of reagent presence or absence of gas bubbles was observed. Presence of gas bubbles indicates positive result and vice versa [21].
2.8.2.3. Oxidase Test
- This test is used to identify microorganisms containing the enzyme cytochrome oxidase (important in oxygen transport chain). It distinguishes between oxidase negative Enteroacteriaceae and oxidase positive Pseudomonadaceae.ProcedureA colony of the organism was smeared on a piece of Whatman (No. 1) filter paper that has been previously soaked with oxidase reagent (containing 1% solution of N, N, N, N - tetra methyl-p-phenylene -amine dihydrochloride in purified water) with the aid of clean grease-free glass slide which had been previously flamed and cooled. Presence or absence of purple colour within 10 seconds was observed [21].
2.8.2.4. Citrate Test
- This test is used for the differentiation of Enterobacteriaceae based on the utilization of citrate as the sole source of carbon. This is the identification test for Klebsiella and Escherichiacoli.ProcedureSimmon's citrate agar was melted with agitation and dispersed into test tubes. The test tubes were autoclaved and allowed to cool and set as slants before inoculating with the pure culture using a wire loop. This was incubated at 37°C for 24 hours. A colour change from green to blue was observed [21].
2.8.3. Antimicrobial Susceptibility Test/ Test for Activity of Crude Extracts and Antiperspirants Formulated Using Agar Diffusion Method
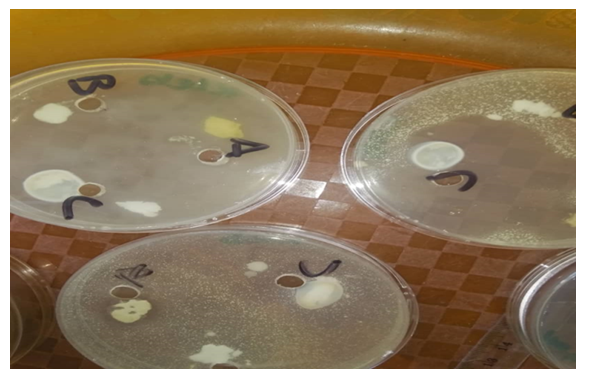
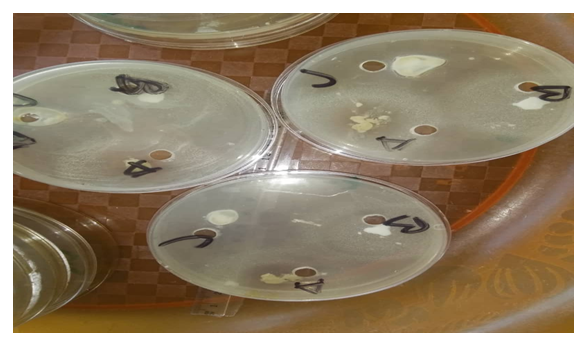
- Sterile Petri dishes were labelled in duplicates for the various test organisms. A 0.1ml of each of the microorganisms was added aseptically to the prepared Muella Hinton Agar pour in the universal bottle and properly mixed. The mixture was then poured into the corresponding Petri dish and allowed to solidify on the workbench. After the agar had solidified on the Petri dish, a sterile cork borer was used to remove 3 discs of agar from the agar layer in order to produce 3 wells in each agar plate. The wells were labelled for two (2) extracts and a control with the third well. Using a separate sterile Pasteur's pipette 0.2ml of the extract was carefully and aseptically added to the well labelled for the extract. 0.2ml of the control was aseptically added into the third well. The Petri dishes were allowed to stay on the workbench for 15 minutes for proper diffusion of the extract and control. The plates (Petri dishes) were incubated at 37°C for 24 hours. The diameter of the resulting Zones of inhibition after incubation around the wells indicatedantimicrobial activity of the extract against the test organisms. They were measured in millimetre (mm) through the base of the plates using a metre rule and recorded [22].
2.8.4. Determination of Minimum Inhibitory Concentration (MIC) of Extracts on Various Microorganisms
- Agar layers containing microorganisms cultured from armpit swab were prepared in various Petri dishes and labelled. Preparation of Different Concentrations; concentration of 100mg/ml of the extract was prepared by dissolving 0.1g of the extract in 1ml of sterile water. Then concentrations of 50mg/ml, 25mg/ml, 5mg/ml, 1mg/ml, 0.5mg/ml, 0.25mg/ml, and 0.125mg/ml were prepared from the stock concentration (100mg/ml) by double dilution procedure. Using sterile cork borers, wells were made and labelled for various concentrations on the agar layer containing microorganisms. 0.2ml of the respective concentrations of the extract were placed into respective bores concentrations. An additional bore was made at the center of the plate and labelled for the control. 0.2ml of the control was placed in the well. The plates were kept on the workbench for 15 minutes to allow the diffusion of the extracts into the agar layer. The plates (Petri dishes) were incubated at 37°C for 24 hours. The diameter of the resulting Zones of inhibition after incubation around the wells indicated antimicrobial activity of the extract against the test organisms. They were measured in millimetre (mm) through the base of the plates using a metre rule and recorded. MIC of extract on the various microorganisms were obtained as less than or equal to the concentrations of the extracts which on further dilution produced no zone of inhibition (activity) on the microorganisms [23].
3. Results
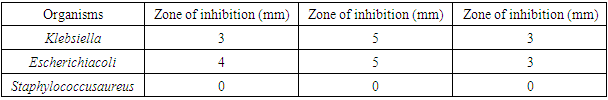
- The minimum inhibitory concentration that has inhibitory effect on the organisms found in the armpit was 5.00% because 5.00% was the least concentration that had an inhibition for all the organisms present. Thus, the reason why the least concentration of crude extract used to formulate was 10.00%.The result for the antimicrobial activity of the antiperspirant formulated with the crude extract at 10.00% against the armpit of the female volunteers showed that for klebsiella, the zone of inhibition was 3mm, 5mm and 3mm in triplicate. The zone of inhibition of Escherichia coli was 4mm, 5mm, and 3mm in triplicate. There was no inhibitory effect observed on Staphylococcus aureus.
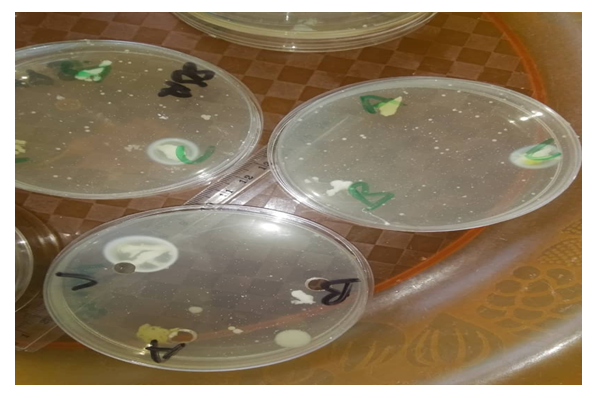
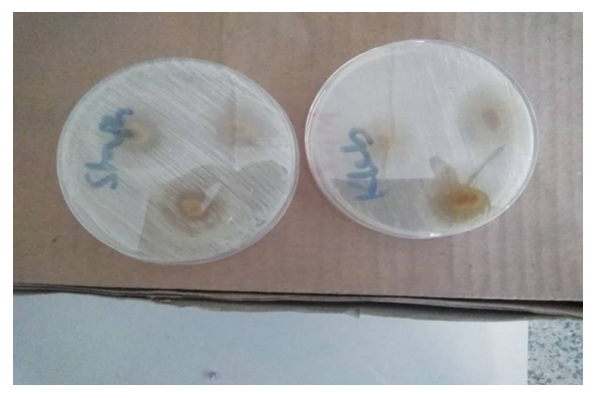 | Figure 4. Nutrient plate showing increased zone of inhibition of antiperspirant with 25.00% crude extract against Staphylococcus aureus and Klebsiella |
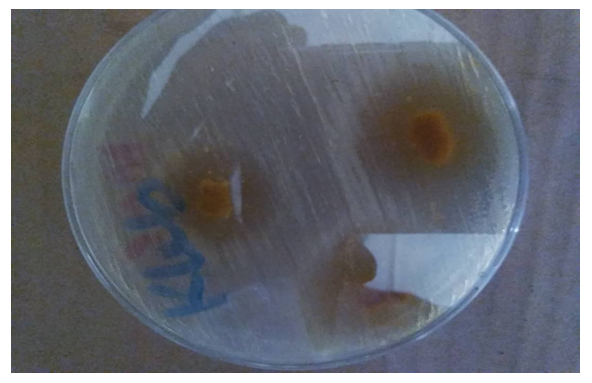 | Figure 5. Nutrient plate showing increased zone of inhibition of antiperspirant with 25.00% crude extract against Klebsiella |
|
|
4. Discussion
- A deodorant is a substance applied to the body to prevent or mask body odour due to bacterial breakdown of perspiration or vaginal secretions, for example in the armpits, groin, or feet [24]. A subclass of deodorants, called antiperspirants, prevents sweating itself, typically by blocking sweat glands. Antiperspirants are used on a wider range of body parts, at any place where sweat would be inconvenient or unsafe, since unwanted sweating can interfere with comfort, vision, and grip. Other types of deodorant allow sweating but prevent bacterial action on sweat, since human sweat only has a noticeable smell when it is decomposed by bacteria [24].The human body produces perspiration (sweat) via two types of sweat gland: eccrine sweat glands which cover much of the skin and produce watery odourless sweat, and apocrine sweat glands in the armpits and groin, which produce a more oily "heavy" sweat containing a proportion of waste proteins, fatty acids and carbohydrates, that can be metabolized by bacteria to produce compounds that cause body odour. In addition the vagina produces secretions which are not a form of sweat but may be undesired and also masked with deodorants [25]. Human perspiration of all types is largely odourless until its organic components are fermented by bacteria that thrive in hot, humid environments. The human underarm is among the most consistently warm areas on the surface of the human body, and sweat glands readily provided moisture containing a fraction of organic matter, which when excreted, has a vital cooling effect [25]. These statements support the human body site (armpit) used in the sample collection of this present study and the increasing need for more safe antiperspirants in the African environment.In the United States, deodorants combined with antiperspirant agents (deodorant antiperspirant) are classified as drugs by the FDA [25,26]. Antiperspirants a +ttempt to stop or significantly reduce perspiration and thus reduce the moist climate in which bacteria thrive. Aluminium chloride, aluminium chlorohydrate, and aluminium-zirconium-compounds, most notably aluminium zirconium tetrachlorohydrex gly and aluminium zirconium trichlorohydrex gly, are frequently used in antiperspirants. Aluminium chlorohydrate and aluminium-zirconium tetrachlorohydrate gly are the most frequent active ingredients in commercial antiperspirants [27]. Aluminium-based complexes react with the electrolytes in the sweat to form a gel plug in the duct of the sweat gland. The plugs prevent the gland from excreting liquid and are removed over time by the natural sloughing of the skin. The good safety that the product of this research work offers is indeed a relative advantage over other deodorant / antiperspirants such as the skin allergy and axillary granuloma response that usually follow deodorants that contain zirconium [28], antiperspirants with propylene glycol, when applied to the axillae, can cause irritation and may promote sensitization to other ingredients in the antiperspirant [29], deodorant crystals containing synthetically made potassium alum were found to be a weak irritant to the skin [30].Meanwhile, it will be worthwhile to further mention here that the common myths and marketing claims related to aluminium compounds in deodorants / antiperspirants include: (a.) that aluminium in deodorants applied to the skin is a risk factor for some cancers (notably breast cancer) and some forms of dementia (Alzheimer's disease). The myth about breast cancer are in two forms – (1) Antiperspirants block the "purging" of toxins which build up in the body and cause breast cancer: There is no truth to this claim. Sweat glands simply do not have this function, and this myth is not scientifically plausible [31]; and (2) Aluminium in antiperspirants can enter the body (possibly through cuts) and cause breast cancer: There is no current evidence to support this claim, nor any convincing evidence that it is true [25,32].
5. Conclusions
- The antiperspirant produced has a better antimicrobial activity against the microorganisms found in female armpits when compared to the antimicrobial effect of the commercial brand of antiperspirant having aluminium has its active agent.
6. Recommendations
- It will be worthwhile to take this research further with a larger sample size, as well as with more different commercial aluminium-containing antiperspirants for a more evidence-based comparison.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML