-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(4): 379-382
doi:10.5923/j.ajmms.20231304.08
Received: Mar. 23, 2023; Accepted: Apr. 9, 2023; Published: Apr. 13, 2023

Multimodal Opioid-Sparing Approach to Postoperative Anesthesia as one of the ERAS Protocol Components
V. Kh. Sharipova, K. Sh. Bokiev
Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the efficiency of multimodal opioid-sparing anesthesia as a component of the protocol for accelerated recovery of emergency patients operated on for peritonitis of various etiologies. Background. Multimodal opioid-sparing pain therapy is the main component of the concept of patients accelerated recovery after various surgical interventions. Adequate pain relief in the postoperative period, based on the principle of multimodal analgesia, promotes early recovery of gastrointestinal peristalsis, reduction of stress response of the body, reduction of the frequency of delirium and cognitive impairment in the postoperative period, early recovery of patients. Material and methods. 203 patients who were treated at the Republican Research Centre of Emergency Medicine with a diagnosis of peritonitis in the period from 2021 to 2022 were examined. The patients were divided into 2 groups: Control group (n=101), who did not use the ERAS protocol and Main group (n=102), who used components of accelerated recovery protocol in the perioperative period and multimodal opioid-sparing technology of postoperative anesthesia. Results. The use of regional technologies under the control of ultrasound navigation as part of multimodal analgesia in emergency patients with peritonitis allowed to reduce the time of the first requirement of anesthesia by 35%, to improve the quality of anesthesia by more than 50%, the duration of anesthesia by more than 2.5 times, which was confirmed by studies of subjective pain assessment indicators on a visual-analog scale. The consumption of narcotic analgesics was noted in the group with the use of opioid-sparing anesthesia technology with a basis consisting of regional anesthesia methods by 67%. Conclusion. Systemic multimodal analgesia was used in both study groups which was supplemented by regional analgesia in the group using the protocol of accelerated recovery after surgery. Accordingly, the reduction in the consumption of narcotic analgesics marked an early recovery of intestinal motility, a decrease in intestinal paresis, which in turn contributed to the early activation of patients, a decrease in delirium manifestations, a decrease in the duration of staying in ICU and hospital stay.
Keywords: Systemic multimodal analgesia, Opioid-sparing therapy, Postoperative pain, Regional anesthesia
Cite this paper: V. Kh. Sharipova, K. Sh. Bokiev, Multimodal Opioid-Sparing Approach to Postoperative Anesthesia as one of the ERAS Protocol Components, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 379-382. doi: 10.5923/j.ajmms.20231304.08.
1. Introduction
- Multimodal opioid-sparing pain therapy is the main component of the concept of patients accelerated recovery after various surgical interventions [1]. Anesthesia of patients after emergency surgical interventions requires a great deal of skill and knowledge from the anesthesiologist, since the individual characteristics of the patient, the state of the body vital functions, the existing water-electrolyte and acid-base disorders must be taken into account. Postoperative pain, analgesia and recovery are factors that cannot be ignored [2]. Adequate pain relief in the postoperative period, based on the principle of multimodal analgesia, promotes early recovery of gastrointestinal peristalsis, reduction of stress response of the body, reduction of the frequency of delirium and cognitive impairment in the postoperative period, early recovery of patients [3,4]. Multimodal analgesia implies opioid-sparing therapy based on the use of regional methods of anesthesia in combination with paracetamol, NSAIDs. Anesthesia of patients should be personalized, patient-oriented, taking into account the trajectory of pain syndrome in the postoperative period [5,6].The aim of the study was to evaluate the efficiency of multimodal opioid-sparing anesthesia as a component of the protocol for accelerated recovery of emergency patients operated on for peritonitis of various etiologies.
2. Material and Methods
- 203 patients who were treated at the Republican Research Centre of Emergency Medicine with a diagnosis of peritonitis in the period from 2021 to 2022 were examined. The patients were divided into 2 groups: Control group (n=101), who did not use the ERAS protocol and Main group (n=102), who used components of accelerated recovery protocol in the perioperative period and multimodal opioid-sparing technology of postoperative anesthesia. The mean age of the patients was 42.1 ± 17.6 years. There were 155 (76.4%) men and 48 (23.6%) women. Patients with acute gangrenous appendicitis were 61 (30%) of the total number of patients. Diffuse purulent-fibrinous peritonitis was observed in 25 (12.3%) patients, local purulent peritonitis was detected in 36 (17.7%) patients. Patients with gastric and duodenal ulcer complicated by perforation were 142 (70%) from the total number of patients (n=203). Of these, diffuse fibrinous-purulent peritonitis was detected in 40 (19.7%) patients, and diffuse serous-fibrinous peritonitis was observed in 102 patients, which was 50.2% of the total number of patients. Appendectomy from McBurney's access was performed in 9 (4.4%) cases, appendectomy by laparoscopic access was performed in 44 (1.7%) patients, appendectomy with laparotomy access was performed in 8 (4%) cases. In peptic ulcer of the duodenum and stomach, complicated by perforation, laparoscopic suturing of the perforated hole was performed in 73 cases (36%). Suturing of the perforating hole by laparotomy was performed in 62 (30.5%) patients, stomach resection by Billrot-II by laparotomic access was performed in 7 (3.4%) cases out of the total number of patients.All patients were performed surgery under general combined anesthesia. The majority of patients in both groups – 47.2% (n=96) corresponded to ASA Class III. Patients corresponding to ASA Class II made up 40% (n=81), ASA Class IV -12.8% (n=26). Scheme of anesthesia: induction in anaesthesia - propofol 2 mg/kg, arduan 0.08-0.1 mg/kg, fentanyl 3 µg/kg. Maintenance of anesthesia - isoflurane 2-2.5 vol% (MAC 1-1.2), fentanyl 2 μg/ kg /hour, arduan according to indications of TOF monitoring (3-4 points). The anesthesia regimen in the main group was supplemented with the use of acytomenophen (paracetamol) 1000 mg and ketoprofen 100 mg as components of multimodal analgesia. At the end of the surgical intervention for postoperative anesthesia, patients of the main group with median-median laparotomy were performed TAP (Transversus abdominis plane block) under the control of ultrasound on both sides with a solution of local anesthetic Bupivacini 0.25% 20 ml on each side with the addition of 4 mg dexamethasone as an adjuvant of local anesthetic. Patients with McBurney access for appendectomy were performed a unilateral TAP block on the right with a solution of local anesthetic Bupivacini 0.25% 20 ml with the addition of 4 mg dexamethasone. During laparoscopic surgical intervention, anesthesia of the trocar injection site was performed with a local anesthetic. Postoperative pain relief was supplemented with NSAIDs and paracetamol. Narcotic analgesics were used as needed. The patients of the control group were performed multimodal anesthesia technology without the use of regional anesthesia methods.The evaluation of pain and the quality of anesthesia in the postoperative period was carried out on the basis of a visual - analog scale (VAS). The time of the first analgesic requirement, the number of narcotic analgesics used, the analysis of the presence of intestinal paresis depending on the number of narcotic analgesics used were also calculated. Statistical analysis was carried out using the StatTech v. 3.0.7 program (developed by Stattech LLC, Russia).
3. Results
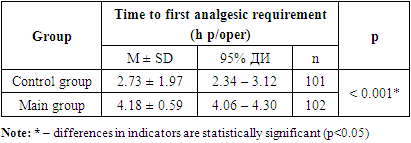
- Studies conducted in the postoperative period revealed that the time to first analgesic requirement in the control group was 2.73 ± 1.97 hours, which was significantly earlier by 34.5% (p<0.001) than in patients of the main group, where this indicator was 4.18 ± 0.59 hours (Tab.1).
|
|
|
|
|
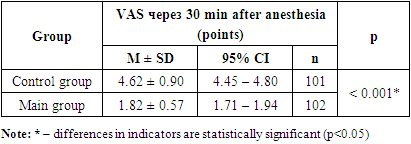
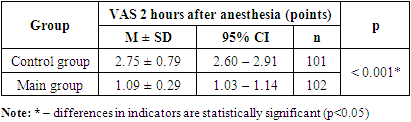
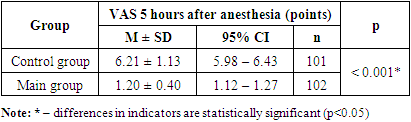
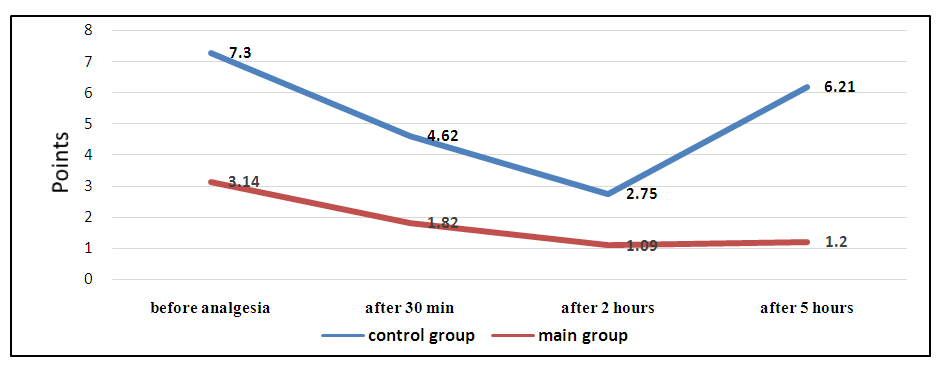 | Figure 1. Dynamics of the indicator of anesthesia quality and duration according to VAS at the study stages |
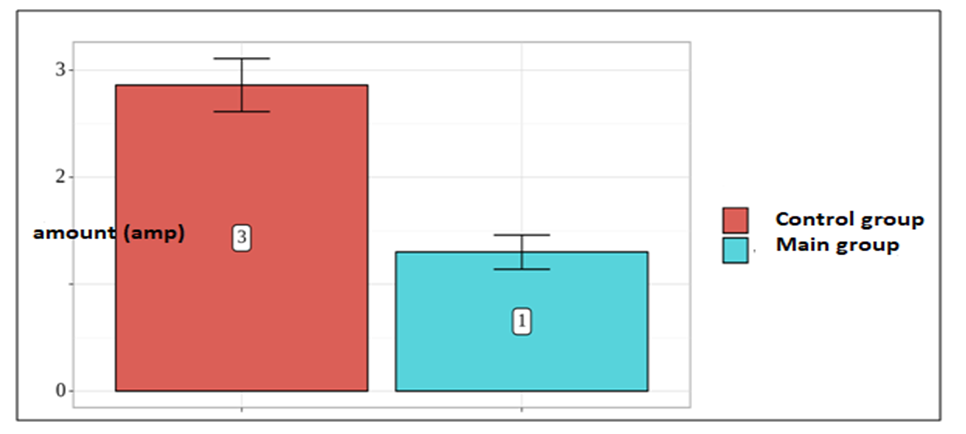 | Figure 2. Comparative analysis of the requirement for narcotic analgesics in the postoperative period |
|
4. Conclusions
- Systemic multimodal analgesia was used in both study groups which was supplemented by regional analgesia in the group using the protocol of accelerated recovery after surgery. Accordingly, the reduction in the consumption of narcotic analgesics marked an early recovery of intestinal motility, a decrease in intestinal paresis, which in turn contributed to the early activation of patients, a decrease in delirium manifestations, a decrease in the duration of staying in ICU and hospital stay.The consumption of narcotic analgesics was noted in the group with the use of opioid-sparing anesthesia technology with a basis consisting of regional anesthesia methods by 67%.The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML