-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(4): 367-373
doi:10.5923/j.ajmms.20231304.06
Received: Mar. 17, 2023; Accepted: Mar. 27, 2023; Published: Apr. 13, 2023

Options for Pectus Excavatum Correction
V. A. Markushin, R. Ya. Khayaliev, Sh. U. Rakhimy
Tashkent Medical Academy, Multidisciplinary international clinic "Surgemed", Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study is to improve the results of pectus excavatum correction due to a differentiated approach to each patient and the choice of the optimal treatment method. Background. The article reveals the problem of treating pectus excavatum. The historical facts on the correction of the pectus excavatum have been given. Modern treatment options were described, advantages and disadvantages of each method were noted, directions for the development of thoracic and plastic surgery for pectus excavatum in the near future were outlined. A large own material on the correction of this deformity using Vacuum Bell was presented for the first time. Material and methods. 2002 patients with pectus excavatum were corrected in the Zdrav clinics, the TS-clinic in Krasnodar and the Surgemed clinic (Uzbekistan, Urgench) for the period 2019-2022. 156 patients of them were citizens of Uzbekistan. There were 1352 males and 650 females. The age of patients ranged from 3 to 52 years. 250 (12.48%) patients were performed the Nuss surgery, in the remaining 1752 (87.52%) cases, the pectus excavatum was corrected using Vacuum Bell. Results. The original method of monitoring the treatment and supervision of the patient until full recovery was described. A universal unified algorithm for collecting information about the patient was developed. The following data are needed to fully understand the essence of the deformation of the child's chest: full name, date of birth, height, weight, chest circumference at the level of the funnel, the distance between the areoles. The duration of wearing the bell varied from 9 months to 36 months. Maceration of the skin during prolonged wearing of the bell is developed in 70% of patients. However, a method of protecting the skin from the edge of the vacuum bell has been developed for this purpose. Conclusion. A comprehensive examination of the patient with an individual approach and determination of the optimal treatment tactics is necessary in the case of pectus excavatum. With timely treatment of patients, especially before the complete formation of the skeleton and the development of gross deformation, Vacuum Bell can completely correct the anomaly that has arisen, relieve a person from both physical illness and psychological problems. The Nuss surgery is necessary for patients with a Haller Index of more than 3.5; older age group (=22 years); lack of efficiency from Vacuum Bell.
Keywords: Pectus excavatum, Plastic surgery, Correction, the Nuss surgery, Vacuum Bell
Cite this paper: V. A. Markushin, R. Ya. Khayaliev, Sh. U. Rakhimy, Options for Pectus Excavatum Correction, American Journal of Medicine and Medical Sciences, Vol. 13 No. 4, 2023, pp. 367-373. doi: 10.5923/j.ajmms.20231304.06.
1. Introduction
- Pectus excavatum and Pectus carinatum are the most common chest deformities. However, the pathogenesis has been poorly studied and the research results remain contradictory. Particular attention is paid to the embryonic and intrauterine development of the ribs and sternum, based on the understanding that the origin of these deformities is due to a violation of the maturation of the parasternal region. Congenital chest deformities can be divided into rare forms such as cleft sternum, Pentalogy of Cantrell (POC), asphyxiating thoracic dystrophy (Jeune syndrome), Poland syndrome and spondylothoracic dysplasia (Jarcho-Levin Syndrome), which account for approximately 3-4% of all cases. Pectus excavatum (PE) and pectus carinatum (PC) account for 95-97% of all chest wall deformities [1]. PE is an indentation in the anterior chest wall resulting from a dorsal deviation of the sternum and third to seventh rib or costal cartilage and is the most common chest deformity accounting for 90% of all cases. Depending on the severity of the PE, deviations of the chest organs and spinal deformities are known. Although PE in most cases practically does not affect the function of internal organs, the cosmetic appearance of patients leads to psychological disorders requiring therapy. Surgical interventions are the most recognized methods of treatment. Various methods of work have been developed. There are several hypotheses regarding the formal pathogenesis of PE, although the underlying pathomechanisms are completely unclear. In addition, questions about the role of development processes in the formation of PE arise. The first description of the pectus excavatum was made by Bauhinus J. in the seventeenth century [2]. Another documented description of the chest appearance can be found in 1860 in Woillez [3]. Von Luschka H. reported a 6 cm deep depression in the chest wall of a 24-year-old man in 1863 [4]. In 1870, Eggel published the first comprehensive report on a patient with funnel-shaped depression in the chest, calling it a "miracle of nature" [5]. He suggested that the cause of the deformity may be weakness and abnormal flexibility of the sternum caused by a nutritional disorder or developmental delay. Individual case reports were submitted by Williams CT., Flash M. and Hagmann [6-8]. Hagmann believed that the overgrowth of the ribs causes chest depression. On the contrary, Langer and Zuckerkandel supported the hypothesis of a developmental disorder occurring in utero, in which the lower jaw of the embryo (fetal mandible) is responsible for deformation by pressing on the sternum as a result of too high intrauterine pressure [9]. As for surgical correction, Meyer L. performed the first PE operation in 1911 with the removal of the costal cartilage [10]. He also analyzed the removed cartilage under a microscope and revealed nonspecific degeneration. However, he did not associate histological data with the pathogenesis of PE.The morbidity of PE has a ratio between 0.1 and 0.8 per 100 people [11]. Interestingly, males suffer more often, with a gender distribution ranging from 2:1 to 9:1. Even if PE occurs sporadically, a genetic predisposition is likely, as a positive family history can be detected in 43% of PE cases. However, a specific genetic defect has not been detected yet. Most cases of PE could be noted clinically during the first year of life, but the primary occurrence during puberty was also described. Most often chest deformities are a single anomaly, but they can also be one of the manifestations of various genetic disorders.Clinically, PE is associated with a typical posture: thin, tall patients with a large abdomen and shoulders pushed forward, which can lead to permanent scoliosis. Compression of the sternum can lead to displacement of the heart and a decrease in lung volume. Anatomical changes occur in chest pain, fatigue, shortness of breath on exertion, respiratory infections, asthma symptoms, palpitations [12]. Coln et al. demonstrated that 95% of 123 patients had cardiac compression. Even one case of syncopal symptoms has been reported [13]. Fonkalsrud EW, et al. reported that the symptoms of many untreated PE patients progress with age and he recommended surgery for both young and adult patients [14].An improvement of pulmonary and/or cardiovascular symptoms and an improvement in subjective well-being after surgical correction were described in the numerous clinical studies. Many authors identify psychological factors due to the physiognomic features of chest deformity. It is believed that deformities cause appropriate social discrimination, especially in adolescence, which leads to socio-psychological problems [15]. A multicenter study showed that surgical treatment of patients with PE improves these socio-psychological issues [16].There are several hypotheses regarding its pathogenesis. Bauhinus put forward the first pathophysiological hypothesis, indicating hypertension of the diaphragm during embryonic development as a pathophysiological factor [2]. In the late nineteenth and early twentieth centuries, one of the leading views on pathogenesis was intrauterine pressure on the sternum due to the abnormal position of the embryo [17]. An alternative idea was in acquired damage caused by constant mechanical stress, which was often found in shoemakers, so the term "shoemaker's chest" appeared. Further hypotheses highlighted other diseases such as syphilis or rickets as the cause of PE. Today's leading hypotheses focus on metabolic disorders in the sternocostal cartilage, which leads to biomechanical weakness and proliferation of sternocostal cartilage. The latter hypothesis was proved by Fokin et al., who found variable cellularity and matrix disorganization in the cartilage of patients with PE [18]. However, Nakaoka et al. demonstrated that the costal cartilage on the side of the deepest impression no longer compared with the cartilage on the opposite side [19].Systematic analysis of histological changes in sternocostal cartilage in patients with PE revealed premature aging of cartilage. Ultrastructural and biochemical studies demonstrated trace element abnormalities in costal cartilage in patients with PE, namely a decrease in zinc levels and an increase in magnesium and calcium levels, which demonstrated that a lack of zinc in the diet leads to a decrease in the metabolic activity of chondrocytes [18]. Feng J, et al. were able to demonstrate a deficiency of biomechanical qualities of cartilage in patients with PE [20]. These data provide interesting information about the correlation of metabolic disorders and mechanical properties of cartilage in PE. Finally, there are two main hypotheses of PE pathogenesis: impaired development or overgrowth of cartilage. Excessive growth caused by maturation disorders is discussed in the last one. Taking into account these two aspects, a potential connection with the development of ribs and the sternocostal joint is of interest.After the first documented surgical correction of PE performed by Meyer, in 1949 Ravitch performed an advanced technique of open intervention [21-22]. Six years later, Rehbein and Wernicke used crossed metal blades during chest stabilization surgery [23]. Open access subperichondrial resection of all deformed costal cartilages, resection of the xiphoid process, and sternal osteotomy with anterior fixation of the sternum with predominantly multiple rod implantation represented the gold standard until the beginning of the last decade. The procedure consists of a median longitudinal incision along the sternum in men and inframammary skin incision in women, resection of the deformed costal cartilages, and complete mobilization of the sternum, which usually requires excessive retrosternal dissection and transverse osteotomy of the sternum. The final shape of the ventral thorax correction is fixed and stabilized with various forms of metal bars in a different number of implanted bars and their positioning, longitudinal and/or transverse, depending on the preference of the surgeon. The surgery time required for open correction ranges from 2 to 5 hours, and perioperative blood transfusion is often required. Besides, pleural drains and multiple wound drains are used in most cases. Patients are discharged from the hospital after 6-8 days. The implanted rods are removed 1 year after surgery during a short stay in the hospital for 2-3 days. In 1998, Nuss et al. introduced a minimally invasive technique as an alternative to standard open repair, the so-called minimally invasive pectus excavatum repair [24]. The Nuss surgery, in which the sternum is lifted with a metal rod behind the sternum under thoracoscopic control, relies on the flexibility of the chest in young patients, allowing effective correction without the need for extensive resection of the costal cartilage or osteotomy of the sternum. Previously, the rod was removed 2 years after the surgery, although in recent years it has been recommended to leave it in place for 3 years.Various minimally invasive approaches are the so-called Erlangen technique and the magnetic mini-motion procedure: the surgery using the "Erlangen technique" is characterized by minimal cartilage resection and reduced anterolateral mobilization. The sternum is mobilized by retrosternal dissection through an anterior incision, and an elastic metal rod is implanted transsternally through suture incisions. Minimal cartilage resection is provided by intraoperative tensiometry. This method measures the forces at regular intervals required to lift the chest and determines if complete separation is necessary. The metal rod will be removed after 1 year [25].Most of PE surgeries methods are based on a stabilizing metal material, which must be removed during the second intervention and it entails several complications. First of all, it is necessary to perform a second intervention. Besides, metal devices can shift and migrate to neighboring tissues. In addition, the injected material can also cause problems such as postoperative chronic pain. From the point of view of the development of new biomedical materials, the use of absorbable materials is of interest, because repeated intervention is not required. Several approaches were performed using absorbable material to correct chest deformities. Torre M. described the use of stabilizers made from Poly(lactic-co-glycolic acid) (PLGA) [26]. Однако Pilegaard HK et Licht PB. published unsatisfactory results with this device: they found a higher complication rate with absorbable stabilizers for the Nuss technique [27].A non-surgical alternative in the treatment of PE is a vacuum bell of various sizes and shapes placed on the patient's chest, negative pressure is applied under the bell using a hand pump every day for several months. Schier F, et al. demonstrated that the vacuum bell may be an alternative to surgical treatment in less severe cases and in preoperative preparation. Haecker published data on 133 patients and confirmed Schier's findings. However, there are no long-term results yet. The aim of the study was to improve the results of pectus excavatum correction due to a differentiated approach to each patient and the choice of the optimal treatment method.
2. Material and Methods
- 2002 patients with pectus excavatum were corrected in the Zdrav clinics, the TS-clinic in Krasnodar and the Surgemed clinic (Uzbekistan, Urgench) for the period 2019-2022. 156 patients of them were citizens of Uzbekistan. There were 1352 males and 650 females. The age of patients ranged from 3 to 52 years. 250 (12.48%) patients were performed the Nuss surgery, in the remaining 1752 (87.52%) cases, the pectus excavatum was corrected using Vacuum Bell (Fig. 1).
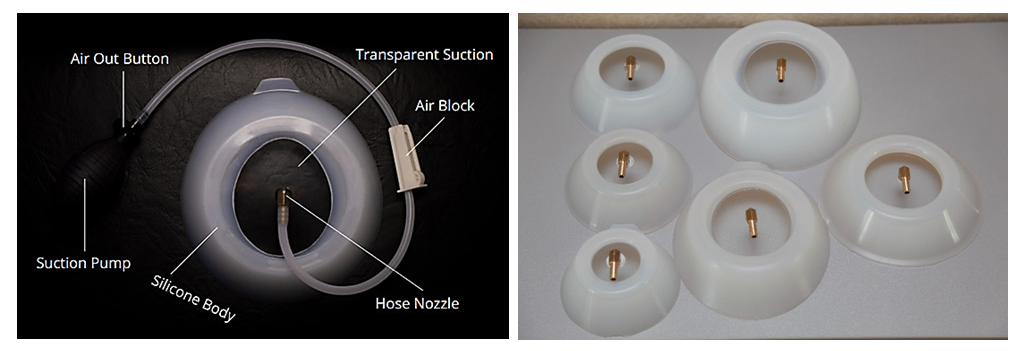 | Figure 1. Vacuum Bell (there are different sizes of bells on the right) |
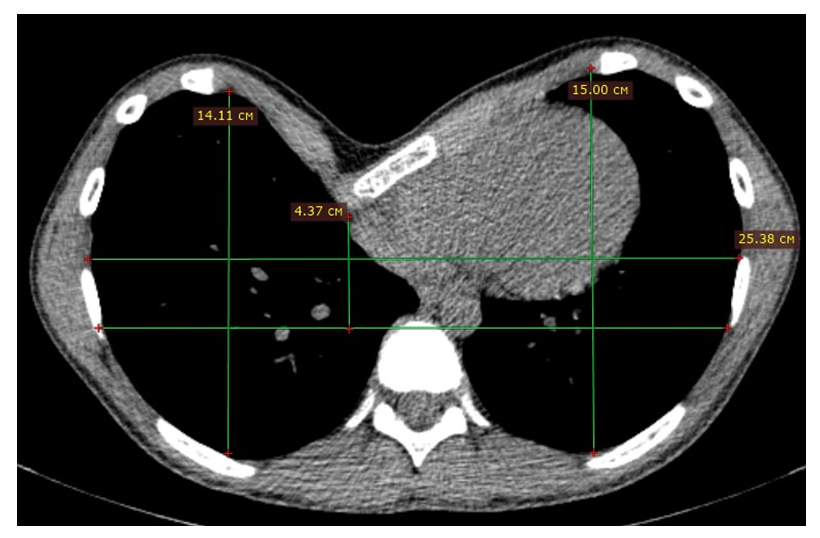 | Figure 2. An example of a MSCT-scan of a patient with PE |
3. Results
- A universal unified algorithm for collecting information about a patient has been developed in the following format. The following data are needed to fully understand the essence of the deformation of the child's chest: full name, date of birth, height, weight, chest circumference at the level of the funnel, the distance between the areoles, MSCT of the chest organs with recording on a disk. Below are examples of photos of a girl with PE before the start of treatment and while wearing a bell (Fig. 3), as well as after 6 months of treatment (Fig. 4).
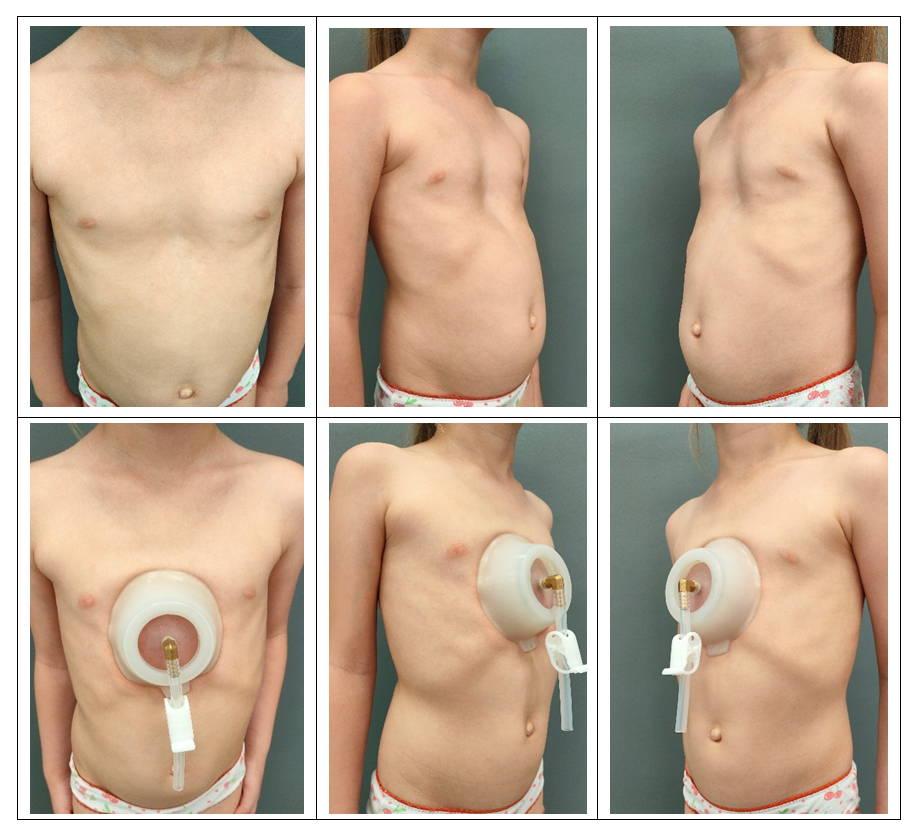 | Figure 3. Photo of a girl with PE before and while wearing a bell |
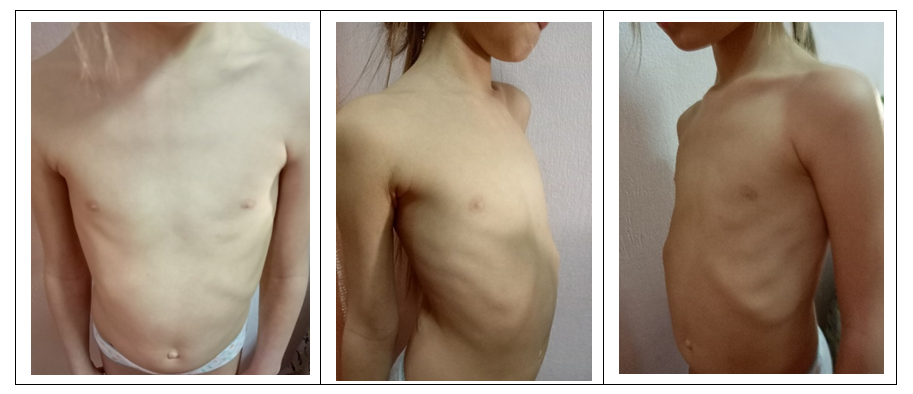 | Figure 4. Photo of a girl with PE after wearing a bell |
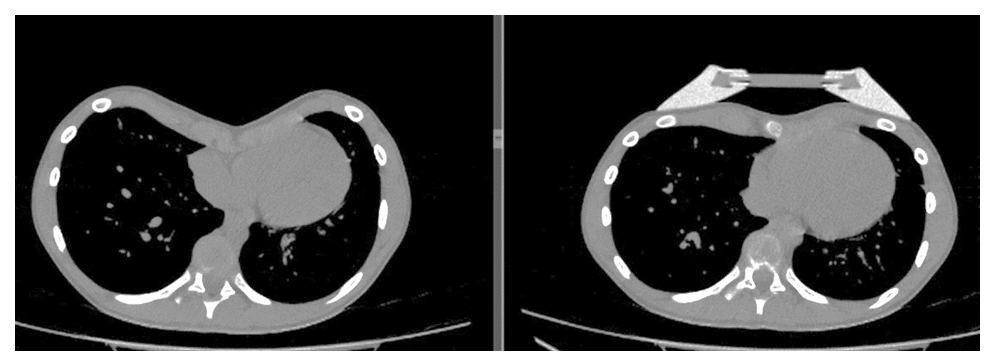 | Figure 5. MSCT-scans before wearing the bell and during the procedure |
4. Conclusions
- At the present stage of the development of medicine, a differentiated approach to the choice of a treatment method for a particular disease is maintained. In the case of Pectus excavatum, a comprehensive examination of the patient with an individual approach and determination of the optimal treatment tactics is necessary. Uncorrected pectus excavatum leads to scoliosis, kyphosis, and cardiorespiratory disorders.With timely treatment of patients, especially before the complete formation of the skeleton and the development of gross deformation, Vacuum Bell can completely correct the anomaly that has arisen, relieve a person from both physical illness and psychological problems. The Nuss surgery is necessary for patients with a Haller Index of more than 3.5; older age group (=22 years); lack of efficiency from Vacuum Bell.In other cases, it is possible to use a bell if the age of the patients does not exceed 25 years. The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML