-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(3): 319-324
doi:10.5923/j.ajmms.20231303.26
Received: Mar. 6, 2022; Accepted: Mar. 20, 2023; Published: Mar. 24, 2023

Assessment Methods for Human Embryos to Enhance Reproductive Potential
Suraya Z. Yuldasheva 1, Oksana V. Shurygina 2
1Associate Professor, Tashkent Pediatric Medical Institute, Uzbekistan
2Doctor of Medical Sciences, Samara State Medical University, Russia
Correspondence to: Suraya Z. Yuldasheva , Associate Professor, Tashkent Pediatric Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Some clinics have invested in cutting–edge technology that allows for continuous video monitoring of embryo development. The approach is based on the employment of unique time–lapse technology, in which each embryo is photographed at a predetermined frequency beginning with fertilization and continuing throughout the incubation phase [7,8,9]. Optimizing the in vitro fertilization program with a unique approach to using time–lapse technology or video monitoring of embryo development. From 2016 to 2019, patient recruitment was carried out on the basis of Closed Joint Stock Company IDC (information diagnostic company) Medical Corporation (Samara, Russia). The Samara State Medical University’s Bioethics Committee approved using human embryos in scientific research. Higher hCG (+)/CPR (clinical pregnancy rate) results were obtained in the study groups using video surveillance, and the difference between these indicators is minimal, indicating a high quality of embryos selected for transfer (IVF 36.7±6%/34.3±7.1% with video surveillance and 42.5±7.4%/36±6.7% without video surveillance). ICSI: 30.1±6.6%/24.1±5% with video surveillance and 35±6.6%/25.3± 4.9% without). Time–lapse technology is considerably more critical in the older reproductive age group (36+ years). The difference in hCG(+)/CPR levels of 34.7±8.1%/30.5±4.6% is slight in the video surveillance group. Analyzing the morphokinetic characteristics of embryo pre–implantation development allows for selecting competent embryos for transfer into the uterine cavity and cryopreservation.
Keywords: Assisted reproductive technologies, Infertility, Elective blastocyst transfer, Time–lapse microscopy, Morphokinetics
Cite this paper: Suraya Z. Yuldasheva , Oksana V. Shurygina , Assessment Methods for Human Embryos to Enhance Reproductive Potential, American Journal of Medicine and Medical Sciences, Vol. 13 No. 3, 2023, pp. 319-324. doi: 10.5923/j.ajmms.20231303.26.
1. Introduction
- With the advent of new assisted reproductive technologies into clinical practice, the likelihood of having children in situations of previously incurable kinds of infertility in marriage has dramatically increased. Assistive technology has advanced over time, and medicine and embryology are now much beyond what was thought in the twentieth century [1]. Competency evaluation of cultured embryos is in high demand since it is a method for selecting an embryo with the best chances of implantation [1]. Some clinics offer modern equipment for continuous embryo growth monitoring, allowing you to watch and analyze the synchronization and rate of development without opening the incubator and removing the embryos outside. The approach is based on the employment of unique time–lapse technology, in which each embryo is photographed at a predetermined frequency beginning with fertilization and continuing throughout the incubation phase. Yet, this is not the sole advantage of time–lapse technology [7,8,9]. During IVF cycles, the embryologist frequently works with many embryos. These embryos differ in that some correspond to the recommended developmental criteria and are, therefore, the most hopeful for implantation in the uterine cavity, while others are less promising and do not correspond to modern views about embryo development. With the introduction of technology for the continuous monitoring of embryos, the embryologist now has access to the most thorough video chronicle of each individual embryo’s early growth. The embryo goes through a number of major events (development stages) during its development, and the time it takes to go from one stage to the next is a crucial signal in determining its quality and implantation potential–development kinetics. Time–lapse technology has added a new tool to the embryologist’s toolbox, allowing for better selection of the most promising embryos and therefore increasing the probability of conception.The objective is to optimize the in vitro fertilization program with a differentiated approach to the use of time–lapse technology or video surveillance of embryo development, which enables the automatic formation of the morphodynamic profile of a human embryo based on video recording of the cultivation of a human embryo to the blastocyst stage.
2. Materials and Methods
- Between 2016 and 2019, patients for the study were recruited at CJSC IDC Medical Corporation (Samara, Russia) throughout the period of 2016 to 2019. The study utilized human embryos per international ethical and legal norms for treating human embryos [Article 18 of the 1997 Council of Europe Convention for the Protection of Human Rights and the Dignity of the Human Being in the Application of Biology and Medicine]. The Bioethics Committee of the Samara State Medical University approved the use of human embryos in scientific study (statement from protocol No. 116 dated October 3, 2018). Participation in the study was authorized by the signed informed consent of all patients. Exclusion criteria included any problems or difficulties necessitating the elimination of embryo transfer (ET) throughout the trial cycle. Patients enrolled in ART programs were subjected to an anamnesis, gynecological examination, laboratory and instrumental investigations. CJSC IDC Medical Corporation implemented ART programs in compliance with the recognised norms of medical care. Under the direction of a stereomicroscope, gametes and embryos were detected (Nicon, Japan). Australia’s COOK incubators were utilized for incubation at 5% O2.Using Time–lapse technology, more than 100 cycles were studied. EmbryoVisor, an incubator with a built–in video camera, is the embodiment of the embryo development video surveillance system (Russia). From 1 to 5–6 days of development, embryos were grown in specific WOW dishes (Vitrolife, Sweden) using the universal medium Continius Single Culture (Irvine Scientific, USA). With this technique, there were no precise criteria for selecting patients for culture. The system is accessible online directly. To evaluate the development of embryos from day 1 to day 5–6 of in vitro cultivation, the time of the first cleavages, the time interval between the first and second cleavages, the nature of cleavage (morphokinetics), and the time of blastocyst formation were considered. All of the aforementioned characteristics served as predictors of embryo selection for transfer. The criteria for elective transfer of one embryo on the fifth day (5eSET) were the presence of more than two embryos of exceptional quality, the patient’s age between 18 and 35 years old, and the lack of any prior IVF attempts. The criteria for selective single embryo transfer (5SET) were the presence of a scar on the uterine wall as a result of previous surgical operations and other clinical circumstances. In the assisted reproductive technologies (ART) laboratory of the Clinical Hospital CJSC IDC “Medical Company” (Mother and Child group of companies, Samara, Russia), the collecting, labeling, and preparation of visual information on cultured human embryos were performed. Markup information and graphic material have been uploaded to the SberCloud cluster. On the Christofari supercomputer of the SberCloud cluster, the convolutional neural network used to solve the multi–class classification issue is implemented. To standardize the description of the development of human embryos cultured in vitro, “Morphodynamic profile of a human embryo” was introduced. It consists of a set of morphokinetic states detected by us and positioned on the time scale based on the moment of their registration. All time thresholds (points) are presented in chronological order relative to fertilization. The acquired information was processed using Microsoft Excel 2007 and STATISTICA 6. For unrelated ranges, the significance of differences in numeric indicators was examined using the Wilcoxon test; the Fisher–Irwin exact test was utilized for qualitative values. At p<0.05, differences between groups were deemed statistically significant; Spearman’s non–parametric rank correlation approach was used for correlation analysis.
3. Results and Discussion
- For non–invasive monitoring of the pre–implantation development of human embryos in the ART laboratory, a multi–gas incubator with a reduced oxygen concentration (5%) and EmbryoVisor (Westtrade, Russia) video surveillance system were utilized. To standardize the description of the development of human embryos cultured in vitro, the idea of “Morphodynamic profile of a human embryo” was introduced in collaboration with the inventors of the EmbryoVisor system. It consists of a set of morphokinetic states detected by us and positioned on the time scale based on the moment of their registration. All time thresholds (points) are presented in chronological order relative to fertilization. Determination of the morphodynamic profile enables the ranking of developing embryos in order to identify the most promising embryo for implantation for transfer into the uterine cavity, as well as embryos for eventual cryopreservation (second stage embryos). On the basis of the identified markers, time–lapse images of human embryo cultivation cycles available to laboratories are marked according to the approved algorithm for preparing data sets for training a neural network, and data is uploaded for subsequent training of a neural network designed for automated recognition of the morphokinetic state of a human embryo cultivated to the blastocyst stage.The following characteristics were incorporated in the profile’s formation:• time of production of pronuclei PN;• time of disappearance of pronuclei;• time of formation of 2, 3, 4, 5, 6, 7, and 8 blastomeres);• time of the beginning of embryo compaction;• time of full compaction of the embryo;• time of the beginning of embryo cavitation;• time of formation of a complete blastocyst;• time of formation of expanded (enlarged) blastocyst;• time of blastocyst hatching.The following additional parameters are utilized to evaluate development dynamics:• proper fertilization (number of observed pronuclei: 1, 2, 3 or more);• the degree of embryo fragmentation (in percent);• the presence of multinucleation (Figure 3);• heterogeneity of the cytoplasm – the presence of endoplasmic reticulum EPR;• heterogeneity of the cytoplasm (inclusions);• vacuolization of embryonic cells;• abnormalities in the shape of the embryo;• the presence of reverse crushing;• uniformity of blastomeres during crushing.Furthermore, the markup system has been updated to meet the criteria imposed by the use of neural network technologies. The EmbryoVisor incubator software’s standard marker configuration mechanism has added more marker groups. Optionally, the researcher creating the cycle markup can place numerous markers on the pronuclear section. We have selected and categorized 612 cycles of human embryo cultivation based on the information we have on the cultivation of more than 2,000 human embryos (a total of 13,367,420 frames). In addition to the states in the morphodynamic profile, the researchers highlighted the principal focus plane (the most informative). In addition, logical constraints were set during employee selection, eliminating plainly unfit candidates. These methods enabled a reduction in the number of frames sent for neural network training to 1,124,937. As part of the generation of the training sample, candidates that spent an abnormal amount of time at any of the developmental stages and embryos with a small number of stages passed were eliminated. In the first stage, the position of the embryo in the microwell is determined using a machine learning model based on the neural network architecture “Faster R–CNN”.This method permits the determination of the effective region of the image for further classification and the verification of the embryo’s real presence in the image. The personnel distribution according to the classification parameters is shown in Table 1.
|
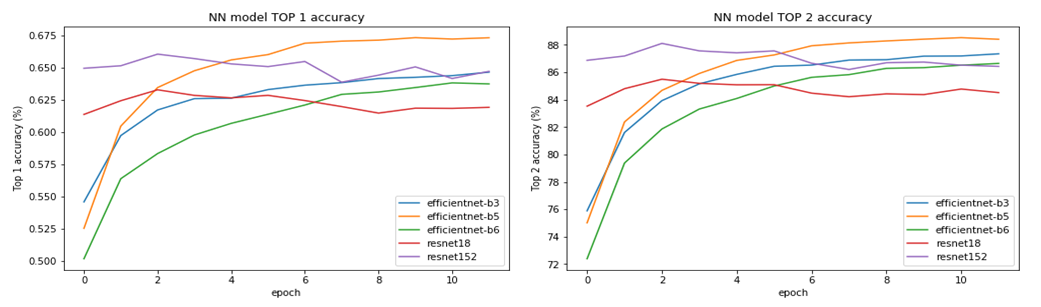 | Figure 1. Comparison of models with different architectures |
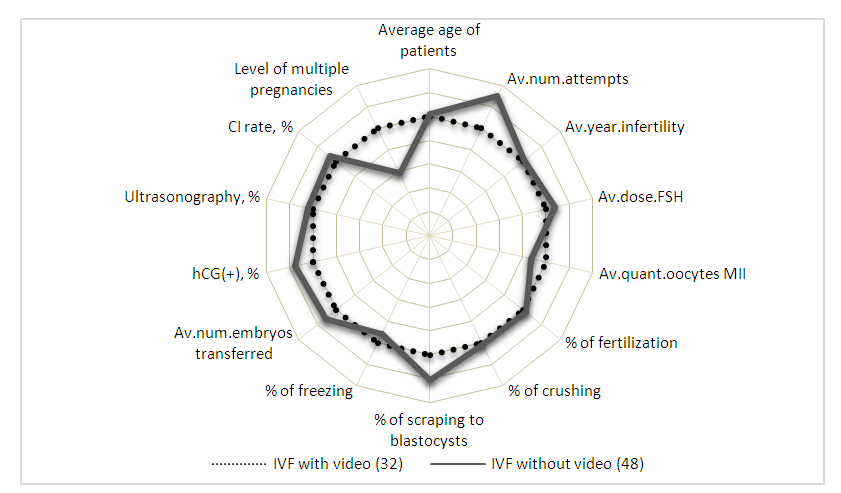 | Figure 2. Comparative characteristics of the developmental indicators of embryos obtained in the IVF program |
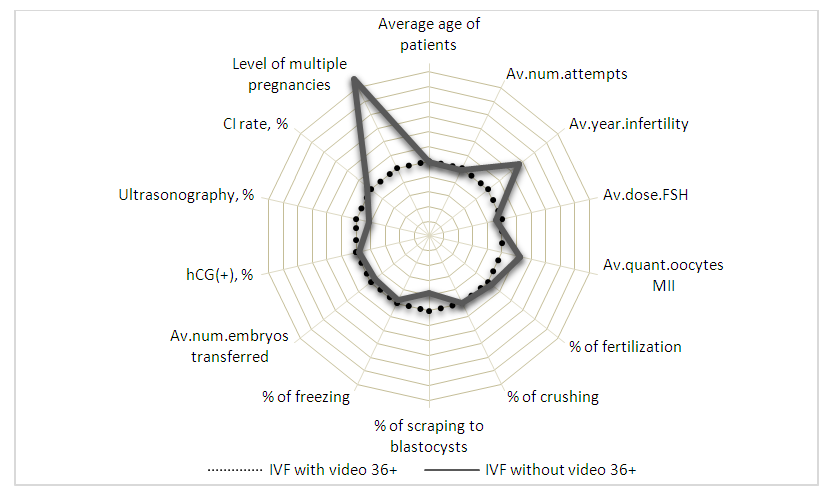 | Figure 3. Comparative characteristics of the developmental indicators of embryos obtained in the IVF program in the age group over 36 years |
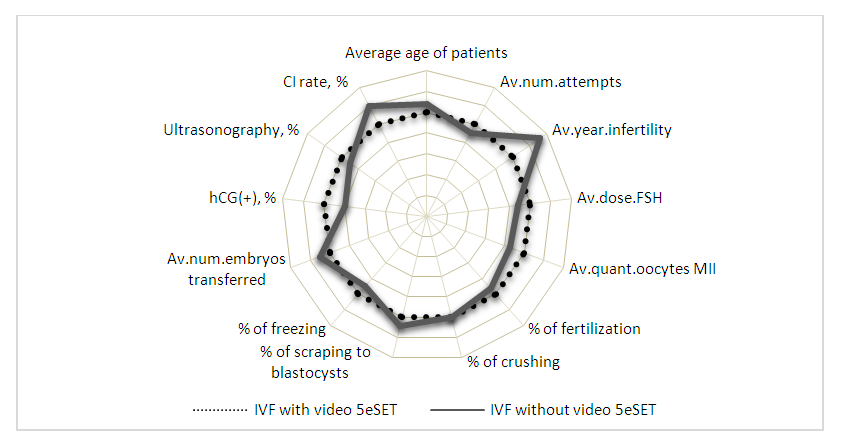 | Figure 4. Comparative characteristics of the developmental indicators of embryos obtained in the IVF program and the transfer of one best embryo |
4. Conclusions
- The video surveillance technology for embryo growth makes it possible to eliminate the effect of the human factor and raise the objectivity of evaluating the structure of embryos, thereby enhancing their selection and decreasing the rates of multiple births.On the basis of the studies undertaken, the fundamental principles of using time–lapse technology have been analyzed and studied. Analyzing the morphokinetic parameters of embryos’ pre–implantation development enables the selection of competent embryos for transfer into the uterus and cryopreservation. The objective selection of the most implantable embryo enables pregnancy, preventing ineffective transfers and shortening the time to conception. Single embryo transfer prevents the birth of premature and underweight infants (during the development of twins and triplets) and minimizes the chance of birth traumas. Such an approach does not necessitate additional public spending for nursing preterm and underweight infants.Consequently, within the study context, the necessity and efficacy of non–invasive time–lapse technology for optimizing assisted reproductive technology programs have been demonstrated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML