-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(3): 311-318
doi:10.5923/j.ajmms.20231303.25
Received: Mar. 1, 2023; Accepted: Mar. 14, 2023; Published: Mar. 22, 2023

Formulation of Antiperspirant Using Crude Extract from Flower Bud of Clove: (Syzgium aromaticum, L. Merrill and Perry)
Pharm. Favour I. Olusanya 1, Professor Ogbonna Okorie 2, Dr Onyenoha Chukwumerije 3, Pharm. Oluwatomi T. Olusanya 1
1Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
2Former Dean, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
3Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
Correspondence to: Pharm. Favour I. Olusanya , Faculty of Pharmaceutical Sciences, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

There is an increasing demand for antiperspirants in the Nigerian market and the need for safe and effective aluminium-free antiperspirants due to the health hazards associated with such. Therefore, this study was conducted to determine if crude extracts of clove (Syzgium aromaticum) containing tannins, could be an effective active ingredient in the antiperspirant formulation. The flower buds of clove were obtained, identified as UPHM313 in the herbarium, washed, dried, powdered and extracted using acetone. The tannin component was further derived using soxhlet extraction technique. Tannins were identified using phytochemical tests followed by preparation of the antiperspirant using different concentrations of the extract; a placebo containing only the excipients was also formulated. The Minimum Inhibitory Concentration (MIC) of the crude extract was determined. Evaluation of the effectiveness of the antiperspirant produced and the placebo was performed via antimicrobial susceptibility testing to the bacteria isolated from the armpits of female volunteers: Staphylococcus aureus, Pseudomonas, Escherichia coli, Klebsiella. The crude tannin extract was shown to have antimicrobial effect, showing increased inhibitory effect with increase in concentration. 10.00% concentration of the crude extract in the formulation was the minimum concentration needed for effective antibacterial activity, and the average zones of inhibition of Staphylococcus, Escherichiacoli and Klebsiella being 0mm, 4mm and 3.7mm, respectively, while at 25.00% concentration, 16mm, 0mm and 27.7mm, respectively. The antiperspirant developed is capable of inhibiting the growth of microorganisms which cause odor in the armpits of humans. There is need to explore this work further for commercial benefits.
Keywords: Formulation of Antiperspirant, Crude Tannin Extract, Flower Bud of Clove
Cite this paper: Pharm. Favour I. Olusanya , Professor Ogbonna Okorie , Dr Onyenoha Chukwumerije , Pharm. Oluwatomi T. Olusanya , Formulation of Antiperspirant Using Crude Extract from Flower Bud of Clove: (Syzgium aromaticum, L. Merrill and Perry), American Journal of Medicine and Medical Sciences, Vol. 13 No. 3, 2023, pp. 311-318. doi: 10.5923/j.ajmms.20231303.25.
Article Outline
1. Introduction
- Antiperspirants are sweating-inhibiting agents. The most important active ingredients used in these preparations are aluminium and zirconium salts. These agents reduce sweat by causing mechanical obstruction to the eccrine sweat duct at the acrosyringium level [1]. Perspiration is a fundamental physiological process whose function is to control and maintain the body temperature at around 37°C.Overproduction of sweat, sweaty skin and body odours are unpleasant for many social groups. Body cleansing products are designed to combat these undesirable features of skin. In addition, antiperspirant and deodorant products are more specifically used in the underarm site by a large part of the adult population. Antiperspirants are offered to control emotionally triggered sweating in the armpit [2]. In addition, it is possible that a proportion of the self-reported deodorant usage is actually antiperspirant usage, as most people think that both deodorants and antiperspirants are the same thing. Whereas deodorants reduce body odor and have an antiseptic action against bacteria, antiperspirants reduce the amount of sweat acting on pores. The overall result is the same in both instances (reducing axillary odor), but the fact that both deodorants and antiperspirants have different mechanisms of action suggests that they possibly have different biological / genetic relationships [3].The terms 'antiperspirant' and 'deodorant' are frequently used interchangeably, although they have quite distinct actions. An antiperspirant actively reduces the amount of underarm perspiration while a deodorant masks and/or reduces odour through the use of an antimicrobial agent or a fragrance. Examples of antiperspirants include aluminum chlorhydrate, aluminium sesquichlorohydrate, aluminium zirconium tetrachlorohydrate, etc. Aluminium salts are used primarily to reduce the wetness, they also reduce the feed stock for bacterial breakdown and they are now known to have recognizable antimicrobial properties [4].An antiperspirant can also be a deodorant, because it can help to control sweat and contain a fragrance at the same time. But deodorants only mask body odor; they don't help to prevent sweating [5]. Deodorants work by targeting those bacteria. Many deodorants contain a chemical called triclosan. It makes your skin too salty or acidic to support bacteria. It will keep you dry, by keeping the bacteria under control [5].Statement of problemMost antiperspirants in the market have aluminum as their active ingredients. However, this material has been linked to different types of cancers, including breast cancer. Some chemical antiperspirants are effective, however, the side effects like allergy and irritations are also obvious. The adverse effects caused by chemical antiperspirants could be avoided by using natural plant extracts. It will be beneficial to develop antiperspirant products that contain safe natural products, such as tannins, as the active ingredients thus replacing products that contain the suspect aluminum substance.Cloves (S. aromaticum) are dried aromatic unopened floral buds of an evergreen tree 10-20 m in height, belonging to the family of Myrtaceae, esteemed as a flavouring agent and also used as a spice for scenting, chewing tobacco and an ingredient of betel chew. [6]. Cloves have many therapeutic uses: they control nausea and vomiting, cough, diarrhea, dyspepsia, flatulence, stomach distension and gastro intestinal spasm, relieve pain, cause uterine contractions and stimulate the nerves [6-9]. In addition, the cloves are highly antiseptic, antimutagenic, antiinflammatory, antioxidant, antiulcerogenic, antithrombotic, antifungal, antiviral and antiparasitic [6,10-15]. Photochemical screening for tannins refers to the extraction, screening and identification of the medicinally active substance found in plants. Some of the bioactive substances that can be derived from plants are flavonoids, alkaloids, carotenoids, tannin, antioxidants and phenolic compounds [16].In the Middle East and Africa, the market for antiperspirants /deodorants is expected to reach USD 783 million by 2023 [6]. Growing westernization among the young population is the primary factor accelerating market growth. Deodorant, which is a lower priced product, is an alternative to fragrances has received strong popularity among Nigerians, especially among the low-income group. Rise in professional young men and women who want to look good to overcome the effect of hot climate in Africa have driven the market. Also, rising consumers demand for natural and organic deodorants in United Arab Emirate (UAE) has significantly affected the market growth [6].Meanwhile, the deodorants market is dominated by international brands, with local alternatives non-existent or insignificant. Therefore, this study aimed at producing an aluminum salts-free, safe and cost-effective antiperspirant for the Nigerian market, using internationally acceptable natural materials and procedure.
2. Materials and Methods
- Materials used included the following: Clove flower buds, Cyclomethicone, Stearyl alcohol, Elaeis guineensis (palm) kernel oil, C12-13 alkyl benzoate, PPG-14 butyl ether, Hydrogenated castor oil, and the Hydrogenated soya bean oil.
2.1. Laboratory Devices Used
- Soxhlet Extraction Method was employed involving the following: Soxhlet extractor, Evaporating dish, Desiccator, Rotatory evaporator, Water bath, and Evaporating dish.
2.2. Plant Material
- Syzgium aromaticum flower bud were obtained from the Mile3 market, Rivers State, Port Harcourt and identified by Dr. Suleiman Mikailu and deposited in the herbarium of Pharmacognosy and Phytotherapy Faculty of Pharmaceutical Sciences, University of Port Harcourt. It was identified as herbarium number UPHM313. The Syzgium aromaticum were washed, then dried. The dried material was ground until it became powdry and subsequently sieved. The powdered material was stored in dried condition until it was extracted with Acetone and the acetone extract obtained.
2.3. Extraction of Tannins from Clove
- Fifty (50) grams of the poweredflower bud of clove was placed into the soxhlet extractor at 56°C boiling point. The solvent was heated to reflux. The solvent vapour travelled up a distillation arm and flooded into the chamber housing the powered clove. The condenser ensured that the solvent vapour cools and drips back down into the chamber housing the powered clove. The chamber containing the powdered clove was slowly filled with warm acetone. The acetone solvent returns back to the distillation flask when the chamber is full. The cycle was repeated many times for 3 days until exhaustive extraction of tannins from clove was achieved. This was done using a Rotarty Evaporator [17] in the Pharmaceutical Chemistry laboratory, Pharmaceutical Sciences, University of Port Harcourt.
2.4. Ferric Chloride Tests
- For the detection of tannins, firstly 5 ml of the extracts filtrate was boiled for 5 minutes along with 5ml solution of 45% ethanol. The mixture was then allowed to cool at room temperature ant was filtered using filter paper. 1 ml of the filtrate was then taken in a test tube and diluted with 2ml of distilled water. Few drops of ferric chloride were then added to the solution. A greenish to black color appeared, indicating the presence of Tannins [16].
2.5. Other Phytochemical Screening
- Alkaloid precipitation, goldbeater’s skin test.
2.6. Preparation of Tannin Antiperspirant
- The emulsions were obtained using the required ingredients that have been weighed / measured according to their calculated values. The fat soluble ingredients for the different emulsions prepared are: Stearyl alcohol, Corn starch, Hydrogenated soya bean oil, Hydrogenated castor oil, C12-13 alkyl benzoate, Palm kernel oil and the water soluble ingredients are: Cyclomethicone, PPG-14 butyl ether and Tannins.
2.6.1. Formulae for the Preparations of the Antiperspirant with Crude Tannin Extract Using 10%, 25% and no Crude Tannin Extract (0%), Respectively
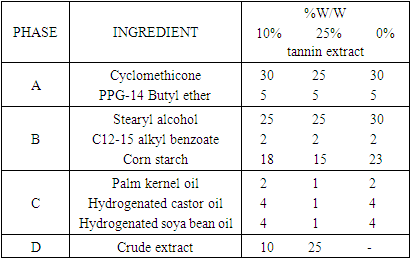 Mixing procedures for 10% and 25% concentrations of the crude tannin extracts were the same as follows: Phase A was heated to 65°C. Phase B was added. it was mixed using an homogenizer; Phase C was added to Phase A + B. mixing was continued for 30mins; Phase D was added to Phase A + B + C and it was mixed until homogenized emulsion was achieved. It was then cooled to 35°C [18]. The mixing procedure of the formula for the preparation of antiperspirant without crude tannin extract was as follows: Phase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizer; Phase C was added to Phase A+B. missing was commenced for 30minutes; Phase D was added to Phase A+B+C and it was mixed until homogenous [18].
Mixing procedures for 10% and 25% concentrations of the crude tannin extracts were the same as follows: Phase A was heated to 65°C. Phase B was added. it was mixed using an homogenizer; Phase C was added to Phase A + B. mixing was continued for 30mins; Phase D was added to Phase A + B + C and it was mixed until homogenized emulsion was achieved. It was then cooled to 35°C [18]. The mixing procedure of the formula for the preparation of antiperspirant without crude tannin extract was as follows: Phase A was heated to 65°C and Phase B was added to it. It was mixed using an homogenizer; Phase C was added to Phase A+B. missing was commenced for 30minutes; Phase D was added to Phase A+B+C and it was mixed until homogenous [18].2.7. Evaluation of Antiperspirant
2.7.1. Collection of Bacterial Isolate
- Four female volunteers were directed not to apply any antiperspirant or deodorant directly to the underarm. After the day’s activity they were taken to the Pharmaceutical microbiology laboratory of Pharmaceutical Science University of Port Harcourt and sterile swabs were taken on the underarm of each of the volunteers The swabs were streaked in the nutrient plates: Nutrient agar, Mac and MSA and incubated for 24hours for microbial growth at 36°C [19].
2.7.2. Microbial Identification
- Microorganisms can sometimes be identified on the basis of how often they appear on or in different media; the pigmentation, size, odour, haemolysis on blood and shape of microbial colonies as they appear on or in the different agar plates [20].
2.7.2.1. Gram Staining
- This is a differential technique used to identify bacteria based on their characteristics either as Gram positive or Gram negative organisms which is based on the ability of cell wall to retain a certain stain or not [21].ProcedureThe isolated organisms from the plates was smeared on a clean glass slide by placing a loopful of water and an inoculum on the slide and allowed to air dry. The smear was then heat fixed and allowed to dry. The prepared smear was flooded with to 1% crystal violet dye for 1 minute, after which the excess stain was rinsed off using distilled water, the smear was then flooded with Lugol’s iodine for I minute, the iodine was rinsed off with distilled water, after which the smear was also flooded with 98% alcohol for 30 seconds, rinsed quickly with distilled water and then Safranin red was added. Allowed for 1 minute and rinsed off with distilled water. The various smear was air dried for few minutes and a drop of oil immersion was added and observed under the light microscope. The same procedure was repeated for the different test results [21].
2.8. Biochemical Test
- The biochemical tests were carried out on the test isolates obtained. They include the following:Catalase TestThis test is used to differentiate bacteria that produce catalase enzyme from those that does not produce the enzyme. The function of the enzyme is to detoxify hydrogen peroxide formed from the superoxide dismutase by breaking it down into water and oxygen gas2H2O2 CATALASE 2H2O + O2ProcedureA drop of 3% hydrogen peroxide was placed on a clean non greasy microscope slide. A flamed and cooled wire loop was used to emulsify a colony of each isolated microorganism on the drop of reagent presence or absence of gas bubbles was observed. Presence of gas bubbles indicates positive result and vice versa [20].
2.8.1. Oxidase Test
- This test is used to identify microorganisms containing the enzyme cytochrome oxidase (important in oxygen transport chain). It distinguishes between oxidase negative Enteroacteriaceae and oxidase positive Pseudomonadaceae.ProcedureA colony of the organism was smeared on a piece of Whatman (No. 1) filter paper that has been previously soaked with oxidase reagent (containing 1% solution of N, N, N, N - tetra methyl-p-phenylene -amine dihydrochloride in purified water) with the aid of clean grease-free glass slide which had been previously flamed and cooled. Presence or absence of purple colour within 10 seconds was observed [20].
2.8.2. Citrate Test
- This test is used for the differentiation of Enterobacteriaceae based on the utilization of citrate as the sole source of carbon. This is the identification test for Klebsiella and Escherichiacoli.ProcedureSimmon's citrate agar was melted with agitation and dispersed into test tubes. The test tubes were autoclaved and allowed to cool and set as slants before inoculating with the pure culture using a wire loop. This was incubated at 37°C for 24 hours. A colour change from green to blue was observed [20].
2.8.3. Antimicrobial Susceptibility Test/ Test for Activity of Crude Extracts and Antiperspirants Formulated Using Agar Diffusion Method
- Sterile Petri dishes were labelled in duplicates for the various test organisms. A 0.1ml of each of the microorganisms was added aseptically to the prepared Mueller Hinton Agar pour in the universal bottle and properly mixed. The mixture was then poured into the corresponding Petri dish and allowed to solidify on the workbench. After the agar had solidified on the Petri dish, a sterile cork borer was used to remove 3 discs of agar from the agar layer in order to produce 3 wells in each agar plate. The wells were labelled for two (2) extracts and a control with the third well. Using a separate sterile Pasteur's pipette 0.2ml of the extract was carefully and aseptically added to the well labelled for the extract. 0.2ml of the control was aseptically added into the third well. The Petri dishes were allowed to stay on the workbench for 15 minutes for proper diffusion of the extract and control. The plates (Petri dishes) were incubated at 37°C for 24 hours. The diameter of the resulting Zones of inhibition after incubation around the wells indicated antimicrobial activity of the extract against the test organisms. They were measured in millimetre (mm) through the base of the plates using a metre rule and recorded [21].
2.8.4. Determination of Minimum Inhibitory Concentration (MIC) of Extracts on Various Microorganisms
- Agar layers containing microorganisms cultured from armpit swab were prepared in various Petri dishes and labelled. Preparation of Different Concentrations; concentration of 100mg/ml of the extract was prepared by dissolving 0.1g of the extract in 1ml of sterile water. Then concentrations of 50mg/ml, 25mg/ml, 5mg/ml, 1mg/ml, 0.5mg/ml, 0.25mg/ml, and 0.125mg/ml were prepared from the stock concentration (100mg/ml) by double dilution procedure. Using sterile cork borers, wells were made and labelled for various concentrations on the agar layer containing microorganisms. 0.2ml of the respective concentrations of the extract were placed into respective bores concentrations. An additional bore was made at the center of the plate and labelled for the control. 0.2ml of the control was placed in the well. The plates were kept on the workbench for 15 minutes to allow the diffusion of the extracts into the agar layer. The plates (Petri dishes) were incubated at 37°C for 24 hours. The diameter of the resulting Zones of inhibition after incubation around the wells indicated antimicrobial activity of the extract against the test organisms. They were measured in millimetre (mm) through the base of the plates using a metre rule and recorded. MIC of extract on the various microorganisms were obtained as less than or equal to the concentrations of the extracts which on further dilution produced no zone of inhibition (activity) on the microorganisms [22].
3. Results
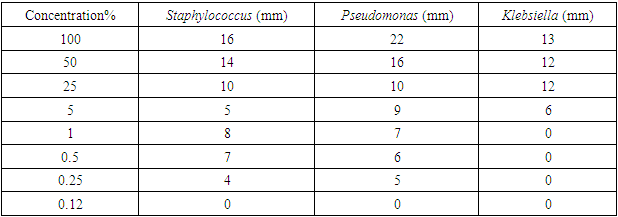
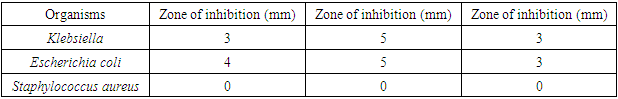
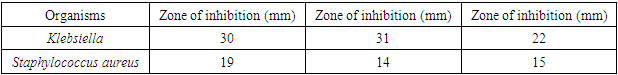
- The microorganisms cultured from the armpit swab for the crude extract test were: Klebsiella, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The results showed increased inhibitory effect with an increase in concentration, using the Minimum Inhibitory Concentration technique (MIC). At 100.00% concentration the crude extract inhibited Staphylococcus, Pseudomonas and Klebsiella by 16mm, 22mm and 13mm, respectively. At 50% concentration, 14mm, 16mm and 12mm, respectively were observed, while at 25%, we got 10mm, 10mm and 12mm, respectively. At 5% concentration, 5mm, 9mm and 6mm, respectively were noted. At 1% concentration, 8mm, 7mm and 0mm, respectively were seen. At 0.5% concentration, the zones of inhibitions were 7mm, 6mm and 0mm for Staphylococcus, Pseudomonas and Klebsiella, respectively. At 0.25% concentration the zone of inhibition was 4mm, 5mm and 0mm for Staphylococcus aureus, Pseudomonas and Klebsiella, respectively. At 0.12% concentration, the zones of inhibitions were 0mm, 0mm and 0mm for Staphylococcus, Pseudomonas and Klebsiella, respectively. The results are summarized in Tables 1 to 4, as well as in Figures 1 and 2 below.
|
|
|
|
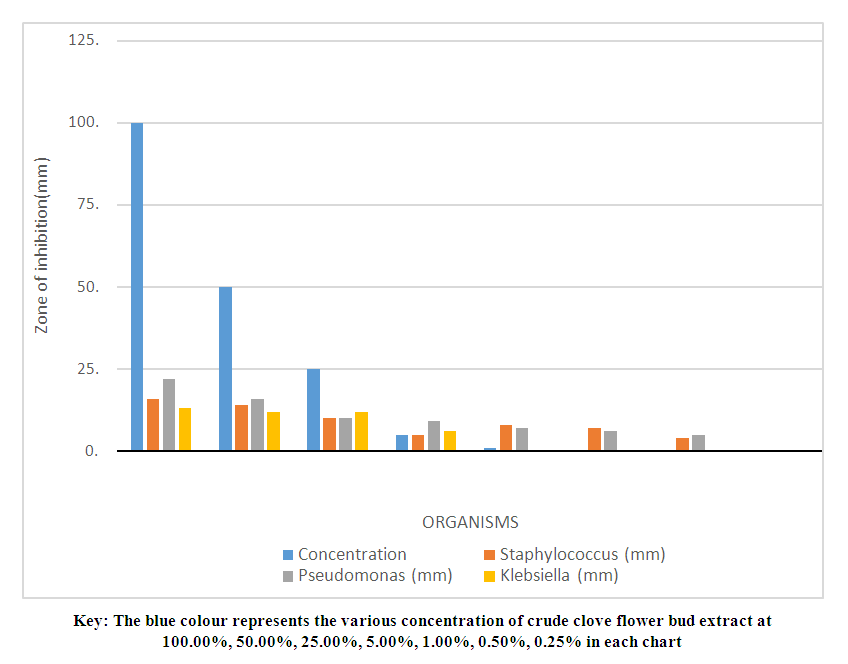 | Figure 1. Antimicrobial test result of crude clove flower bud extract alone |
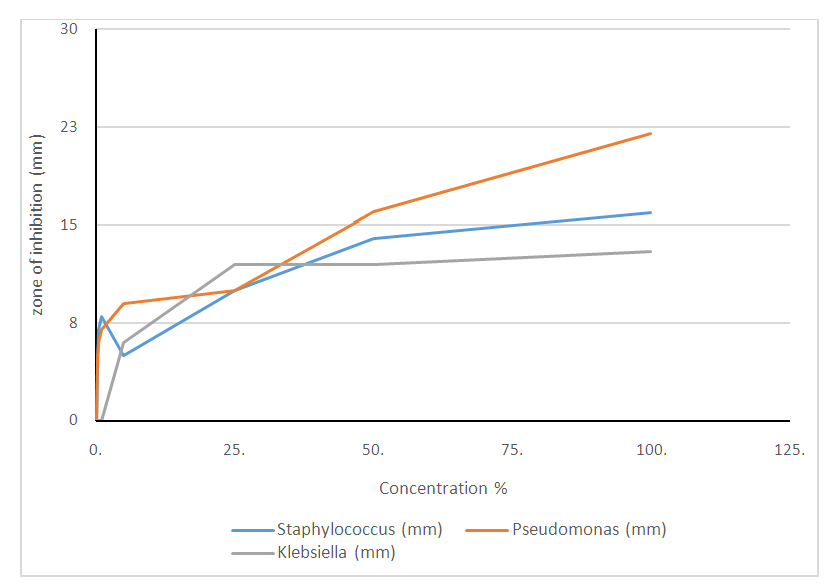 | Figure 2. Antimicrobial test result of crude clove flower bud extract alone at 25.00%, 50.00%, 75.00% and 100.00% concentrations, respectively |
4. Discussion
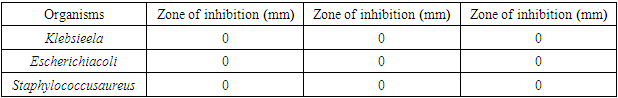
- The crude clove flower bud extract of clove (Szgygiumaromaticum) is shown to have antimicrobial effect against the microorganisms found in the armpit of female volunteers. The microorganisms were Pseudomonas, Staphylococcus aureus and Klebsiella. The minimum inhibitory concentration that had inhibitory effect on the organisms found in the armpit was 5.00%, meaning that 5.00% was the least concentration that had an inhibition for all the organisms present. Thus, the reason why the least concentration of crude extract used to formulate was 10.00%. This is consistent with antimicrobial susceptibility testing [21]. The result of lack of the antimicrobial activity of the placebo against the armpits of the female volunteers was the confirmation of the fact that Klebsiella, Esherichia coli and Staphylococcus aureus actually were inhibited by the active ingredient present in the crude clove buds extract and not by mere chance as only the excipient was in the placebo formulation administered for control. An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts (thus often referred to as "bulking agents", "fillers", or "diluents"), or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerns such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. Many excipients have more than one use, which can an advantage since it reduces the number of excipients needed and minimises the risk of interactions between them [23].Extraction method and solvent play an important role in the extraction of tannins [16]. Soxhlet extraction is a good method when compared to cold maceration. The present Nigerian study used the soxhlet extraction method with acetone as the solvent. The effectiveness of soxhlet extraction is mainly based on the evaporation temperature and polarity index of the solvent. Also, in this present study, the correct evaporation temperature of 56°C was used beside the Synder’s solvent polarity index of acetone (5.4). The suitable temperature of extraction improves the diffusion ratio of tannins into the solvent and the circulation of the solvent [16]. The solvents such as water or ethanol-water mixtures are used for the extraction of the active hydrolysable tannins (gallic acid and ellagic acid), but extract yield of tannins is the lowest with n-hexane [16]. However, soxhlet method needs long duration and high amount of solvent, hence the loss of efficiency of economic and the environmental problems [24]. When the evaporation temperature of the solvents is high, thermal destruction of tannins can happen [25]. Extraction time is about 3 h or 6 h due to the materials [24]. Meanwhile, there are other methods of tannins extraction such as maceration [16], decoction method [26,27], pressurized hot water extraction [28], ultrasound-assisted extraction [29,30], microwave-assisted extraction [31,32], ionic liquid-based microwave-assisted extraction [32], infrared-assisted extraction [33,34], supercritical fluid extraction [35], enzyme-assisted extraction [36], and gamma-assisted extraction [37].
5. Conclusions
- The antiperspirant developed is capable of inhibiting the growth of microorganisms which cause odor in the armpits of humans. Furthermore, the astringent property of the crude extract makes it a good sweat-absorbing agent thus blocking sweat pores in the armpit, which in effect suppresses proliferation of microorganisms.
6. Recommendations
- Further work is recommended on the isolation and characterization of tannins contained in the crude extract. Furthermore, the appropriate environment and instrument needed to expand this work and possibly commercialize the product after due processes have been followed.
Conflicts of Interest
- The authors declare that there is no conflict of interest regarding the publication of this paper.
Source of Funding
- The authors did not receive any funding for this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML