-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(3): 300-303
doi:10.5923/j.ajmms.20231303.22
Received: Feb. 20, 2023; Accepted: Mar. 10, 2023; Published: Mar. 15, 2023

Prognostic Value of NO-Synthase Gene T786C Polymorphism and Angiotensin-Converting Enzyme (ACE) Gene I/D Polymorphism in Patients with Comorbid Diseases of the Cardiorespiratory System Who Underwent COVID-19
Ermekbaeva A. U.1, Kamilova U. K.2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Republican Specialized Scientific-Practical Medical Center of Therapy and Medical Rehabilitation, Tashkent, Uzbekistan
Correspondence to: Kamilova U. K., Republican Specialized Scientific-Practical Medical Center of Therapy and Medical Rehabilitation, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of our study was to prognostic value of NO-synthase T786C polymorphism and angiotensin-converting enzyme (ACE) gene I/D polymorphism in patients with comorbid diseases of the cardiorespiratory system who underwent COVID-19. In 134 patients with COVID-19 have been studied the NO-synthase gene T786C polymorphism and ACE gene I/D polymorphism. The T/C genotype of T786C polymorphism of the NO-synthase gene is characterized by a 1.6-fold increase in the risk of developing comorbid conditions ((χ2=4.3; P=0.04) and can be considered an independent genetic marker as-sociated with the severity of the disease in patients who have undergone COVID-19. It has been established that the D allele and D/D genotypic variants of the ACE gene can be registered as a genetic factor in the negative course of the disease in patients who have undergone COVID-19. Thus, the I/D polymorphism of the ACE gene with a functionally negative D allele and the D/D genotype can be considered an important genetic determinant of an increased risk of de-veloping comorbid conditions and an unfavorable course of the disease in patients who have undergone COVID-19.
Keywords: COVID-19, Endothelial NO synthase gene, Angiotensin-converting enzyme gene, Comorbid diseases
Cite this paper: Ermekbaeva A. U., Kamilova U. K., Prognostic Value of NO-Synthase Gene T786C Polymorphism and Angiotensin-Converting Enzyme (ACE) Gene I/D Polymorphism in Patients with Comorbid Diseases of the Cardiorespiratory System Who Underwent COVID-19, American Journal of Medicine and Medical Sciences, Vol. 13 No. 3, 2023, pp. 300-303. doi: 10.5923/j.ajmms.20231303.22.
1. Introduction
- Coronavirus disease 2019 (COVID-19) is still an ongoing pandemic worldwide. COVID-19 is an age-related disease with a higher risk of organ dysfunction and mortality in older adults. Coagulation disorders and thrombosis are important pathophysiological changes in COVID-19 infection [1]. Up to 95% of COVID-19 patients have coagulation disorders characterized by an elevated D-dimer, a prolonged prothrombin time, a low platelet count and other laboratory abnormalities. Thrombosis is found in critical cases with an increased risk of death [2,3]. Endothelial cells are prone to be affected by the novel SARS-CoV-2 and express angiotensin-converting enzyme 2. The evidence, such as the presence of the virus, has been identified, leading to the inflammation and dysfunction [4,5]. Endothelial cell activation and dysfunction play a pivotal role in the hypercoagulation status in COVID-19 patients. In addition to the direct exposure of subendothelial tissue to blood, Weibel-Palade bodies within the endothelium containing coagulants can be released into the circulation. Endothelial nitric oxide synthase may be impaired, thus facilitating platelet adhesion. Moreover, anti-β2-glycoprotein I antibodies may also contribute to the coagulopathy in COVID-19 by inducing the upregulation of proinflammatory mediators and adhesion molecules [6]. To conclude, coagulation disorders and thrombosis are vital and predict a poor outcome in COVID-19 patients, especially in severe cases. Endothelial cell activation and dysfunction may play an important role in causing clot formation. More basic and clinical research is warranted to further our understanding of the role of coagulopathy and their possible mechanism in COVID-19 patients [7]. The aim of our study was to prognostic value of NO-synthase T786C polymorphism and angiotensin-converting enzyme (ACE) gene I/D polymor-phism in patients with comorbid diseases of the cardiorespiratory system who underwent COVID-19.
2. Materials and Methods
- In 134 patients with COVID-19 have been studied the genetic determinants of alleles and genotypes T786C of endothelial NO synthase gene and ACE gene I/D polymorphism. The control group consisted of 102 healthy individuals. The study was performed according to the standards of Good Clinical Practice (Good Clinical Practice) and the Declaration of Helsinki. The study protocol was approved by the ethics committees of all participating clinical centers. Before inclusion in the study all participants provided written informed consent. 81 of the examined patients (36.8%) suffered from chronic obstructive pulmonary disease (COPD). Their mean age was 56.3±0.94 years. Among the patients, 45 (55.6%) women and 36 (44.4%) men. Among the complaints during hospitalization, cough was noted in 23 (28.3%) patients, chest pain in 28 (34.6%), shortness of breath in 41 (50.6%). Chronic bronchitis was noted in 92 (46%) patients, bronchial asthma in 33 (15%) patients.When analyzing the results of a study of the frequency of comorbid diseases of the cardiovascular system after 6 months of observation in patients who underwent COVID-19, arterial hypertension (AH) was detected in 121 (55%) patients, of which 1/3, i.e. 74 (33.6%) of patients were obese, 83 (37.7%) patients suffered from coronary heart disease (CHD) and 41 (18.6%) patients with chronic heart failure (CHF). Chronic kidney disease (CKD) was somewhat less common - in 19 (8.6%) patients, and atrial fibrillation (AF) - in 21 (9.5%) of the study participants.Study of endothelial NO synthase gene polymorphism T786C and ACE gene I/D polymorphism was conducted using polymerase chain reaction on programmable thermocycler CG-1–96 «Corbett Research» (Australia) and 2720 «Applied Biosystems» (USA), using kits LLC “Medlab” (St. Petersburg), according to the manufacturer’s instructions. Evaluation of deviation of the distribution of genotypes of studied polymorphisms of DNA from the canonical distribution of Hardy-Weinberg equilibrium (HWE) was performed using the computer program for the analysis of genetic data “GenePop” (“Genetics of Population”). To calculate the “odds ratio” (OR — odds ratio) with 95% confidence intervals (CI— confidenceinterval), χ2 and pvalues used statistical package statistical software package «OpenEpi 2009, Version 2.3».
3. Results
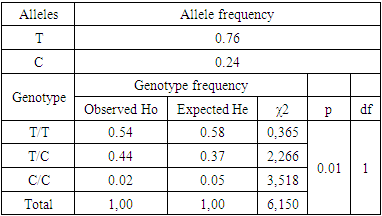
- The endothelial NO synthase gene is an important enzyme responsible for the synthesis of NO in the endothelium and is involved in the regulation of blood vessel tone, the activity of the smooth muscles of the vascular wall, and the processes of thrombosis. Genetic determinants of the development of endothelial dysfunction - according to the T786C polymorphism of the endothelial NO-synthase gene, in 118 patients with COVID-19, the results showed that T alleles accounted for 75.8%, C alleles - 24.2%. In the group of healthy individuals, T alleles accounted for 85.3%, C alleles - 14.7%. The T/T genotype of the T786C promoter was found in 54.1%, the T/C genotype in 43.6%, and the C/C genotype in 1.3% of COVID-19 patients (Table 1).
|
4. Discussion
- Clinically, COVID-19 patients are mostly characterized by respiratory symptoms and disorders. However, coagulation disorders are quite common in COVID-19 patients and sometimes result in thromboses in respiratory, cardiovascular, and venous systems. Coagulopathy not only causes vessel occlusion but also predicts a poor outcome in COVID-19 patients [8]. Additionally, COVID-19 is clearly an age-related disease, and older people are at a higher risk. The mortality rate of elderly patients is 15% higher than that of young patients [9].Endothelium can generate and secrete factors influencing the coagulation system such as heparin cofactor 2, factor V, factor VIII, protein S, protein C, thrombomodulin, tissue factor, vWF and plasminogen activator inhibitor. Endotheliopathy has been observed in SARS-CoV-2 patients [10]. COVID-19 has even been suspected to be an endothelial disease because of its complication profile, such as thrombosis, hypertension, renal failure, and diabetes. In fact, both endothelial activation and dysfunction develop in COVID-19 patients and contribute to their coagulation disorders and thrombosis [11].Endothelial cell activation can be triggered by inflammatory mediators in COVID-19 patients. Endothelial dysfunction is also characterized by a modified endothelial function in the nitric oxide synthase (NOS) system, endothelial tension, and any other alteration of endothelial cells. There are 3 kinds of genes regulating NO in the human body. They are neuronal NOS (nNOS), cytokine-inducible NOS (iNOS) and endothelial NOS (eNOS). eNOS generates endothelium-derived NO and is essential for endothelial function. It is known that endogenous NO has the ability to prevent platelets from adhering to the vascular endothelium [79]. Studies in humans and animals have proven the antithrombotic effects of eNOS, and NO is generated by endothelial cells and platelets [12].Although COVID-19 is primarily a respiratory disease, it has a significant impact on the cardiovascular system, and vice versa, a dysregulated cardiovascular system appears to exacerbate COVID-19. Particularly COVID-19 patients with CVD frequently develop more severe and complicated disease course than patients without CVD. A common denominator of CVD is the dysfunction of vascular cells, increased permeability, endothelial-to-mesenchymal transition, inflammation, and coagulation. It has been assumed that clinical complications are due to SARS-CoV-2 infection of ECs through the ACE2 receptor pathway [13].
5. Conclusions
- The T/C genotype of T786C polymorphism of the NO-synthase gene is characterized by a 1.6-fold increase in the risk of developing comorbid conditions ((χ2=4.3; P=0.04) and can be considered an independent genetic marker associated with the severity of the disease in patients who have undergone COVID-19. It has been established that the D allele and D/D genotypic variants of the ACE gene can be registered as a genetic factor in the negative course of the disease in patients who have undergone COVID-19. Thus, the I/D polymorphism of the ACE gene with a functionally negative D allele and the D/D genotype can be considered an important genetic determinant of an increased risk of developing comorbid conditions and an unfavorable course of the disease in patients who have undergone COVID-19.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML