-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 143-146
doi:10.5923/j.ajmms.20231302.24
Received: Feb. 1, 2023; Accepted: Feb. 17, 2023; Published: Feb. 22, 2023

Toxicological Evaluation of Bacillus Subtilis Probiotic
Alisher M. Urinov, Ilkhom I. Otajonov
Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Alisher M. Urinov, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Probiotics are live bacteria, which, when administered in adequate amounts, have a positive effect on macroorganisms, qualitatively changing the composition of the intestine. The purpose of this study is the toxicological evaluation of the biologically active additive Probiotic BST. The studies were carried out on small laboratory animals in accordance with the current regulatory and methodological framework. The conducted tests showed that dietary supplements for food Probiotic BST belong to the IV class - low-toxic substances, do not have a cumulative, irritating and sensitizing effect, cause dystrophic, necrotic and inflammatory changes in the organs of animals.
Keywords: Probiotic, Laboratory animals, Biochemical and morphological composition of blood
Cite this paper: Alisher M. Urinov, Ilkhom I. Otajonov, Toxicological Evaluation of Bacillus Subtilis Probiotic, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 143-146. doi: 10.5923/j.ajmms.20231302.24.
Article Outline
1. Introduction
- Probiotics are live bacteria, which, when administered in adequate amounts, have a positive effect on macroorganisms, qualitatively changing the composition of the intestine [2]. For more than 50 years, probiotics have been used in medicine in the form of dietary products, biologically active food supplements or drugs.Achievements of modern biotechnology and nutrition convincingly testify to the importance of the microbiome in life processes, the possibility of using biotechnological products in the form of pro-, pre- and metabiotic therapy for the prevention and complex treatment of biliary tract dysfunction, intestinal infections of various etiologies, dysbacteriosis and other common diseases of the gastrointestinal tract [3].To adjust and improve the quantity and quality of the intestinal microflora, probiotics occupy a special place, exerting beneficial effects on the functional functions of the body, the biochemical and behavioral response of the body, optimizing the microecological status of the body [1].Among the modern methods of correcting dysbacterial interruptions in the intestinal microflora, probiotics still occupy the most important. That with the diversification of the statistical data of their law enforcement, it is preferable that publications have increased, that the positive effect of probiotics on dysbacteriosis has a short-term ethos. By itself, one of the most important ineffective probiotic therapy is considered by a large number of authors to be heterogeneous for humans, which are part of their microorganisms [4,5,6,7].
2. Purpose of the Research
- Toxicological assessment of dietary supplements "Probiotic BST" was carried out with intragastric administration in the estimated toxic dose to laboratory animals, followed by observation during the experiment to identify clinical signs of intoxication.
3. Materials and Methods
- Experimental studies were carried out on small laboratory animals (white rats and mice, guinea pigs) in accordance with the current regulatory and methodological framework. To determine the effect of the study drug, toxicological and cumulative studies were carried out, irritant effects on the skin and mucous membranes of the eyes, sensitizing effects, and necropsy were evaluated.Experimental tests were carried out in compliance with the rules adopted by the European Convection for the Protection of Vertebrate Animals for Experimental or Other Scientific Purposes (ETS No. 123. Strasbourg, 18.03.1986).Biochemical blood tests were performed on a semi-automatic biochemical analyzer "CYANSmart" with software (Cypress Diagnostics, Belgium) according to standard methods (ACT, ALT, ALP, total protein - reagent kits Cypress Diagnostics, Belgium), hematocrit was determined on a hematocrit centrifuge (Cypress Diagnostics, Belgium). Belgium), A detailed analysis of peripheral blood was determined in the Goryaev chamber.For the experiment "Probiotic BST" - the contents of the capsules, tablets (the tablets were crushed in a laboratory mortar) were administered in the form of an aqueous solution or suspension. The solvent is distilled water. For intragastric administration of large doses, fractional administration was used.Experimental animals received the same dose in mg/kg per body weight of the object of study within the hours of observation (16-20 hours), the control received an adequate dose of distilled water. Animals were fed 3 hours after dosing. To assess the effect on the mucous membranes, it was injected into the conjunctival sac of m. pigs (the right eye was an experiment, the left eye was a control) [6,7,8,9,10,11].All surviving animals were euthanized at the end of the study by induction into deep anesthesia, after macro- and microscopic morphological studies. The results obtained were subjected to statistical processing using standard programs with an assessment of the significance of indicators (M±m) and differences according to Student's t-test and methodological recommendations "Using the principles of evidence-based medicine in the organization and conduct of hygiene research" based on Word 2010. Differences in the compared groups were considered significant at a significance level of 95% (p<0.05) [12,13,14,15].
4. Results and Discussion
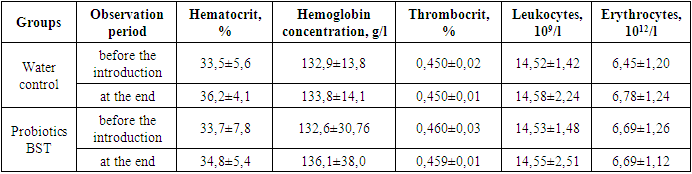
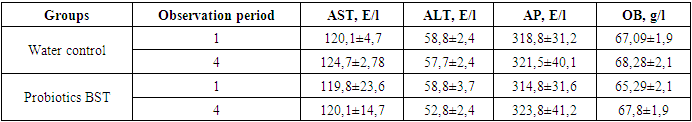
- Under experimental conditions, the establishment of acute toxicity of the studied dietary supplements to food "Probiotic BST" was carried out on 2 types of laboratory animals (white outbred rats and mice) with a single intragastric intake of each name of the drug at doses of 1500, 3000 and 6000 mg / kg of animal weight. During the period of the experiment, the death of experimental animals was not observed. The picture of intoxication was absent throughout the standard observation period. Since it was not possible to establish the average lethal dose of the studied dietary supplements for food "Probiotic BST" for animals taken in the experiment, based on the observational data, dietary supplements for food "Probiotic BST" can be classified as practically non-toxic (class V harmless according to hygienic classification) and low-hazard (hazard class IV according to GOST 12.1.007) substances.A single inoculation of 0.05 ml (2 drops) of solutions or suspensions of the studied dietary supplements for food "Probiotic BST" was carried out - into the conjunctival sac of the right eye of a guinea pig (3 animals in each group). Under the influence of the subjects of dietary supplements, there was no hyperemia, lacrimation or blepharospasm. The average group total score for the severity of mucosal irritation after the termination of contact was 0 points in all samples. Therefore, the obtained research data showed that the dietary supplement "Probiotik BST" - in doses of use does not irritate the mucous membrane of the eye and, according to the severity of the action, belong to the 0 hazard class - no irritant effect on the mucous membranes (Iir-0 points).The cumulative ability of the studied dietary supplements to food "Probiotic BST" was determined by the method of Lim et al. on white rats weighing 110-120 g. Aqueous solutions of the studied dietary supplement were administered intragastrically for 28 days. The initial dose was the recommended daily dose, successively increased every five days by 1.5 times, the control animals were injected with an equivalent volume of distilled water. Over the entire period of observation, the experimental animals did not show any deviations in behavior in comparison with the animals of the control group: they were active, neat, their appetite was preserved and they responded adequately to an external stimulus. There were no signs of intoxication and death. Observation of changes in the weight of rats showed the same increase in weight, and the degree of weight gain did not differ between the experimental groups and the control. In the study of hemodynamic parameters of the peripheral blood of animals, significant changes were not revealed in the studied parameters (Table 1).
|
|
5. Conclusions
- Thus, daily intragastric administration for 28 days in an increasing dose of dietary supplement Probiotic BST does not cause lethal effects, does not lead to changes in physiological parameters, does not cause dystrophic or destructive changes in parenchymal organs and is not accompanied by irritation of the mucous membranes of the gastrointestinal tract. Food supplements "Probiotic BST" do not have the ability to accumulate and are non-toxic.The conducted tests showed that dietary supplements for food "Probiotic BST" belong to the IV class - low-toxic substances, do not have a cumulative, irritating and sensitizing effect, cause dystrophic, necrotic and inflammatory changes in the organs of animals. Therefore, the results obtained allow us to conclude that the dietary supplement "Probiotic BST (Probiotic BST)" with repeated intragastric intake into the body does not have a systemic and general toxic effect, is, according to toxicological indicators, they comply with safety requirements.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML