-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 112-118
doi:10.5923/j.ajmms.20231302.18
Received: Nov. 28, 2022; Accepted: Jan. 30, 2023; Published: Feb. 22, 2023

Effect of Proton Pump Inhibitors on Hepatic Encephalopathy in Cirrhotic Patients with Concomitant Gastroduodenal Disorders
Ziyadullaev Shukhrat Khudoyberdievich1, Bekmuradova Makhsuda Salkhidinovna2, Toirov Doston Rustamovich2
1Doctor of Medical Sciences, Head of Department Internal Medicine №1 of Samarkand State Medical University, Samarkand, Uzbekistan
2Assistent of the Department Propaedeutics of Internal Diseases, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Ziyadullaev Shukhrat Khudoyberdievich, Doctor of Medical Sciences, Head of Department Internal Medicine №1 of Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The most common and worsening the quality of life of patients with liver cirrhosis are neurological and neuropsychiatric disorders, referred to as hepatic encephalopathy (HE). Proton pump inhibitors (PPIs) are commonly prescribed drugs for cirrhosis of the liver accompanied by gastroduodenal disorders. The aim of this study was to evaluate the effect of proton pump inhibitors on hepatic encephalopathy in patients with cirrhosis of the liver with concomitant gastroduodenal disorders. There were 86 study patients in total. We divided them into 2 groups: patients with cirrhosis of the liver with gastroduodenal disorders who received complex therapy with the inclusion of PPIs (Group 1) and patients with liver cirrhosis who took complex therapy without PPIs (Group 2). Basically, to assess the degree of HE among those taking and not taking PPIs, we selected several diagnostic research methods that mainly determine the patient's neuropsychiatric disorders. In patients with liver cirrhosis, in order to determine the speed of cognitive activity, we chose a number connection test and to examine the accuracy of fine motor skills - a line test, from additional research methods, we chose electroencephalography and the level of ammonia in the blood. We conducted an analysis between the two groups to elucidate potential risk factors for the development of HE, as well as medication intake during the follow-up period. In all analysis groups, we found significant differences between PPIs use and the occurrence of HE. According to the result of the number connection test in the 1st group, after treatment, the time spent on performing the test increased by about 6.5 seconds. The time spent to complete the test lines on average increased by 3.1 seconds from the average, and the errors made after treatment were on average 2.6 errors more. According to the results of the EEG in the 1st group, who received complex therapy with the inclusion of drugs from the PPIs group, there was a slowdown in the activity of the α-rhythm by an average of 2.2 frequencies per second and an increase in flashes of θ-waves on the EEG, that is, the degree of hepatic encephalopathy progressed after the treatment. Detoxification function of the liver, assessed by the content of ammonia in the blood serum. During therapy with PPIs in group 1, there was an increase in serum ammonia by an average of 8.7 mg/dL. The comparative analysis of the obtained results of psychometric and psychological testing has shown that in the group of patients who received complex therapy with PPI group (group 1), there was a significant worsening, while in group 2 there was an improvement. Our study showed that a higher probability of developing PE in patients with cirrhosis was observed in group 1, i.e. in the group that used PPIs.
Keywords: Liver cirrhosis, Hepatic encephalopathy, Proton pump inhibitors, Gastroduodenal disorder, Psychometric tests
Cite this paper: Ziyadullaev Shukhrat Khudoyberdievich, Bekmuradova Makhsuda Salkhidinovna, Toirov Doston Rustamovich, Effect of Proton Pump Inhibitors on Hepatic Encephalopathy in Cirrhotic Patients with Concomitant Gastroduodenal Disorders, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 112-118. doi: 10.5923/j.ajmms.20231302.18.
Article Outline
1. Introduction
- The problem of liver cirrhosis today is one of actual problems not only of modern hepatology, but also of all internal medicine [1]. The relevance of this problem lies in its prevalence among individuals of relatively young, working age, the possibility of a number of formidable complications, frequent disability, disappointing results of treatment and relatively high mortality [3,10]. A relatively common complication of cirrhosis in the development of liver failure is neuropsychiatric impairment, termed hepatic encephalopathy (HE) [9]. Patients with cirrhosis are very often at risk of developing hepatic encephalopathy (PE), mainly due to the ammonia produced by the typical gut flora, and may subsequently be at risk for changes in this condition if the microbiome changes in any way [2,3,13]. In cirrhosis, HE can develop by increasing fibrosis at certain stages. But there are additional factors that may accelerate its development or worsen its severity. Well-known triggers for HE include infections, excessive use of diuretics, gastrointestinal bleeding, constipation and drugs used in neurology and psychiatry, such as opioids and benzodiazepines [5,6]. Recently, other etiological factors, such as altered intestinal flora and overgrowth of small-bowel bacteria, have been increasingly mentioned in new studies [23,30]. In recent years, it has become widely known that cirrhosis can affect several organ systems, such as the cardiovascular system, respiratory system, kidneys and bone system [14,24]. Cirrhosis has also been associated with varying degrees and changes in the gastrointestinal tract. In addition to the presence of structural changes in the gastrointestinal tract, such as esophageal varices and portal hypertensive gastropathy, which can cause gastrointestinal bleeding and anaemia, intestinal sensorimotor and barrier function disorders have also been described in cirrhosis [26]. Overall, up to 80% of patients with cirrhosis have one or more related gastrointestinal symptoms [24,26]. The most common gastrointestinal symptoms reported were bloating in 49.5% of patients, abdominal pain in 24%, belching in 18.7%, diarrhoea in 13.3%, and constipation in 8% [24]. The above data call for the use of PPIs in the treatment of cirrhosis with gastroduodenal disorders. Proton pump inhibitors (PPIs) are used by 46-78% of patients with cirrhosis, so it is important to clarify the risk profile associated with these drugs [31]. They are often prescribed for a wide range of cirrhosis-associated gastrointestinal diseases, such as dyspepsia, gastroesophageal reflux, gastric and duodenal ulcers and for the prevention of bleeding from the GI tract [30,36]. In turn, prolonged and indiscriminate use of PPIs reduces gastric juice production and leads to bacterial overgrowth in the small intestine. This leads to excessive ammonia production and thus to the development of HE.
2. The Aim of the Study
- To evaluate the effect of proton pump inhibitors on hepatic encephalopathy in patients with liver cirrhosis and concomitant gastroduodenal disorders.
3. Materials and Methods
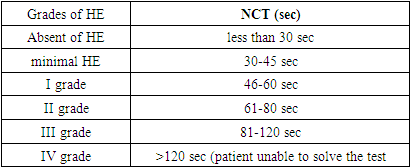
- The study was conducted at the Department of Propaedeutics of Internal Diseases of the Samarkand State Medical University in the Department of the 1st Therapy. We studied 86 patients. The mean age of the patients was 43.5 years. By gender, 48 (55.81%) patients were male and 38 (44.19%) were female.Patients included in the study were:1. patients diagnosed with cirrhosis of any etiology;2. patients with Child-Pugh class A, B, C liver cirrhosis complicated by hepatic encephalopathy and without it. 3. patients with liver cirrhosis accompanied by gastroduodenal disorders and without it.4. Patients with cirrhosis who have and have not taken PPIs.The exclusion criteria used in the selection of patients were:1. patients with neuropsychiatric disorders not due to liver disease.2. patients with severe somatic diseases (diabetes mellitus, renal disease, decompensated heart disease).3. patients who have a history of taking psychotropic drugs.4. Patients with alcohol and drug intoxication.5. Patients with primary and secondary brain tumors.All patients were diagnosed with liver cirrhosis based on clinical signs, imaging and on the basis of liver ultrasound, elastography, liver fibroscan. All participants underwent the following research methods, including:1. A survey was conducted containing subjective information;2. Clinical and neurological examination;3. Study of the neurological condition: using psychometric tests, conducting a question and answer session with the patient. Determination of the degree of HE on the West-Haven scale.4. Cognitive disorders and depression were determined using the MMSE test, the Beck scale.5. EEG;6. Transcranial dopplerography;7. MRI of the brain;8. Laboratory research methods: Complete blood count and general urinalysis; Biochemical analysis of blood (bilirubin, ALT, AST, alkaline phosphatase, ammonia content, creatinine, urea, amount of sodium in the blood, etc.). Clinical and neurological examination consisted of a standard therapeutic and neurological examination and was aimed at identifying the syndrome of HE in liver cirrhosis. Consultation of a neurologist and a psychiatrist was carried out to exclude other causes of encephalopathy. Neurological symptoms were assessed: tremor of the fingers, paresthesia of the extremities, increased tendon reflexes, changes in gait, changes in handwriting, etc. And also, interviews were held with relatives of patients about the dynamics of changes in character and behavioral reactions. All data obtained were recorded in an individual patient card, developed on the basis of the requirements of the study. The severity of HE was determined by the subjective and objective parts of hospital admission records using the West-Haven criteria. The result of the study was an assessment of the severity of HE in people taking PPIs, compared with people not taking them, at the time of admission to the hospital and after the course of treatment. Basically, to assess the degree of HE among those taking and not taking PPIs, we selected several diagnostic research methods that mainly determine the patient's neuropsychiatric disorders. In patients with liver cirrhosis in order to determine the speed of cognitive activity, we chose a test for the connection of numbers and for examination of the accuracy of fine motor skills - a line test, from instrumental research methods - electroencephalography and the level of ammonia in the blood.The number connection test (NCT) and the line test are the most widely used, the sensitivity of which in diagnosing HE reaches 80% [1]. When performing number connection test, the subject must, as quickly as possible, connect the numbers from 1 to 25 with each other in order, within 30 seconds. The time spent on correcting errors was taken into account in the overall evaluation of the results.
|
4. Results
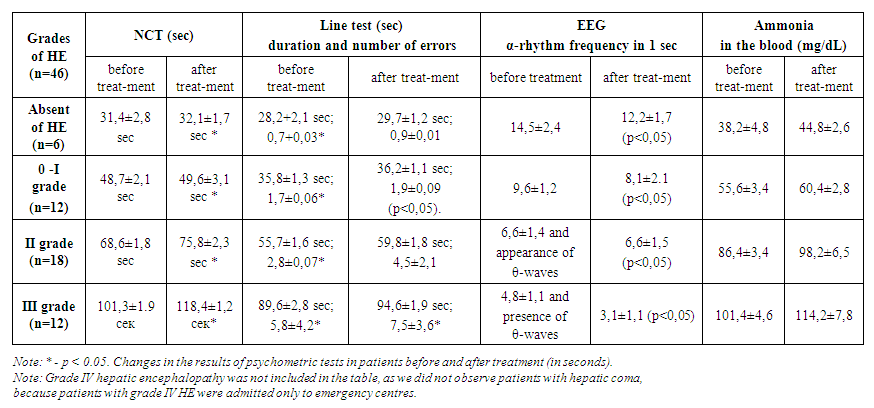
- The study included 86 patients. All patients had a confirmed diagnosis of liver cirrhosis based on imaging studies or liver ultrasonography, elastography, liver fibroscan, and evidence of portal hypertension based on clinical signs, imaging, or portal pressure measurement. Forty-six of these cirrhotic patients were taking PPIs (Group 1) because they had comorbid gastroduodenal disorders. The remaining 40 patients with liver cirrhosis did not take PPIs (Group 2). The main method for diagnosing and evaluating the dynamics of HE in the study was the use of psychometric testing using the number connection test and the line test, EEG and the determination of ammonia in the blood. All patients underwent psychometric testing, EEG, plasma ammonia levels before and after treatment.Assessment of HE before and after treatment in patients with cirrhosis with gastroduodenal abnormalities treated with proton pump inhibitors (1-group)n=46
 | Table 1 |
 | Table 2 |
5. Discussion
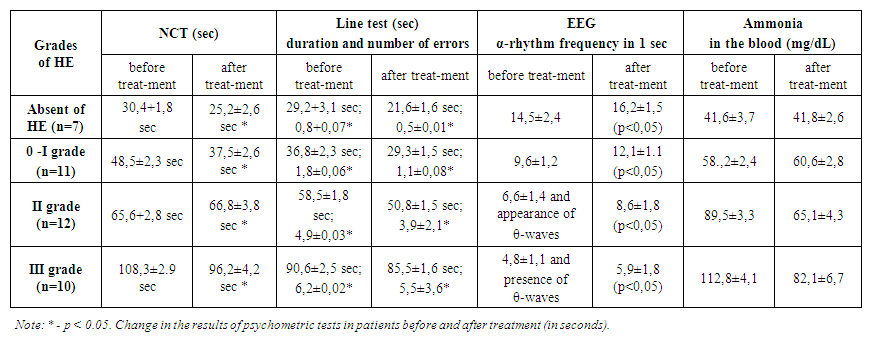
- In general, we believe that an increase in gastric pH promotes more active proliferation of intestinal bacteria. Increased proliferation is not without consequences, as the gut microbiome is one of the body's leading producers of ammonia and therefore may make patients more susceptible to HE. This study showed an association between PPI use and the risk of HE in patients with liver cirrhosis. PPI use has been associated with a significantly increased risk of hepatic encephalopathy in patients with liver disease. Comparative analysis of the results of psychometric and psychological testing has shown that in the group of patients receiving complex therapy with the inclusion of PPI group drugs (group 1), there was a significant deterioration. According to the results of the number connection test in Group 1 after treatment the time required to perform the test increased by approximately 6.5 seconds. The time required to complete the test lines increased with an average of 3.1 seconds, and the errors made after treatment were 2.6 more on average. Analysis of the results of the psychometric examination methods showed that the time required for the number connection test and the line test, as well as the number of errors in the line test, increased significantly in the patients in group 1. EEG findings in group 1 receiving complex therapy including PPIs showed an average 2.2-frequency per second slowdown of α-rhythm activity and increased θ-wave flashes on EEG, i.e. the degree of hepatic encephalopathy progressed after the treatment. Detoxification function of the liver as assessed by serum ammonia content. An increase in serum ammonia by an average of 8.7 mg/dL was observed in group 1 against a background of PPI therapy. In group 2, the results of psychometric and psychological testing showed a significant improvement in the group of patients who received complex therapy. The number connection test in Group 2 after treatment decreased the time required to complete the test by approximately 7.8 seconds. The time required to complete the test lines decreased with an average of 8.1 seconds, and the average number of errors after treatment was 3.4 less. Analysis of the results of psychometric tests in the 2nd group showed that the time required for the number connect test and the line test, as well as the number of errors during the line test, significantly decreased for the patients after the treatment (Table 2). Change in psychometric test performance in patients before and after treatment (in seconds). Note: * - p<0.05.According to the conducted HE therapy, according to EEG data, there was an increase in the amplitude of the electroencephalogram wave, an increase in the frequency of the α-rhythm and a decrease in the flashes of θ-waves, indicating normalization of brain function.The detoxification function of the liver, assessed by the content of ammonia in the blood serum, also normalized after therapy in group 2. In patients of this group, there was also a more pronounced decrease in the level of ammonia in the blood serum, significantly correlating with an improvement in clinical and psychometric indicators (p<0.05). We conducted an analysis in various groups to determine potential risk factors for the development of HE, as well as taking medications during the follow-up period. In all analytical groups, we found significant differences between the use of PPI and the occurrence of HE. Our study showed that a higher probability of developing HE in patients with liver cirrhosis was observed in group 1, that is, in the group using PPIs. This dictates that in patients with cirrhosis of the liver concomitant gastroduodenal pathology should alert practitioners and make them pay attention to the safety of long-term therapy of PPIs. Changes in the intestinal flora after the use of PPIs may be one of the main factors leading to HE in patients with liver cirrhosis. Therefore, among patients with liver cirrhosis, one should carefully approach the determination of indications for the initiation and continuation of PPI therapy, especially for patients with cirrhosis who already have a high risk of developing HE. Further scientific research is needed to confirm our findings.
6. Conclusions
- 1. In conclusion, proton pump inhibitors are often used without regard to their side-effects in hepatology. Our study has shown that PPIs use in cirrhotic patients is associated with a more severe degree of HE compared to those who did not take PPIs. 2. We suggest that PPIs use in cirrhotic patients should be reduced as a means of reducing episodes of HE and suggest their use on strict indications and in low doses in cirrhotic patients with gastroduodenal disorders. 3. Our study showed that PPI use in cirrhotic patients is associated with a more severe degree of HE compared to those who did not take them. This finding suggests that concomitant gastroduodenal disorders in patients with cirrhosis should alert practitioners to the safety of long-term proton pump inhibitor therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML