-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 95-98
doi:10.5923/j.ajmms.20231302.14
Received: Dec. 29, 2022; Accepted: Feb. 3, 2023; Published: Feb. 15, 2023

Pathomorphological Changes Developing in the Renal Arteries and Microvessels under COVID–19 Influence
S. A. Shakirov1, R. I. Israilov2, A. R. Mamataliev3
1Senior Teacher, Andijan State Medical Institute
2Director of the Republican Pathoanatomical Center of the Ministry of Health of the Republic of Uzbekistan
3Head of the Department, Andijan State Medical Institute
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lesion in the large arteries of the kidney, in the arcuate artery between the cortex and the medulla in a greaterdegree as well as hypertrophy of intima cells in the wall of the afferent artery of capillaries, appearance of lymphohistiocytic infiltration, proliferation of endothelium in the wall of the diverting artery are usually observed in case of COVID–19. In some cases the microvessels of the capillary network are significantly dilated and filled, but in other cases are spasmed, the basal membrane of capillaries becomes mucoid, endothelial, podocytic and mesangial cells are proliferated.
Keywords: Covid–19, SARS–CoV–2 virus, ASE2, Somatic diseases, Blood vessels, Complications, Kidneys, Necrotic nephrosis
Cite this paper: S. A. Shakirov, R. I. Israilov, A. R. Mamataliev, Pathomorphological Changes Developing in the Renal Arteries and Microvessels under COVID–19 Influence, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 95-98. doi: 10.5923/j.ajmms.20231302.14.
Article Outline
1. Topicality
- Due to the influence of renin–angiotensin–aldosterone system chain, the development of reactions such as cytokine storm as well as ischemia and hypercoagulability SARS–CoV–2–induced kidney injury occurs. Because of APF2 enzyme is highly expressed in the kidney, endothelium, tubular epithelium, and podocytes of the renal blood vessels can be directly infected by SARS–CoV–2 virus. Virus–induced damage to the vascular endothelium causes their inflammation, increased discirculatory processes, the development of thrombosis causes ischemia and infarction of tissues and organs and diapedetic hemorrhages.
2. Aims and Objectives of Study
- Investigation of anamnesis and autopsy protocols of those who died from COVID–19; carrying out retrospective analysis; studying the patho–morphological changes occurring in the kidney vessels.
3. Materials and Methods
- Anamnesis and autopsy reports data of 86 patients who died from COVID–19 and examined at the RPAC in spring and summer 2021 have been analyzed. At autopsy, pieces of organs were cut and frozen for 72 hours in a formalin solution prepared in 10% phosphate buffer, stained by hematoxylin–eosin method.
4. Results and Discussions
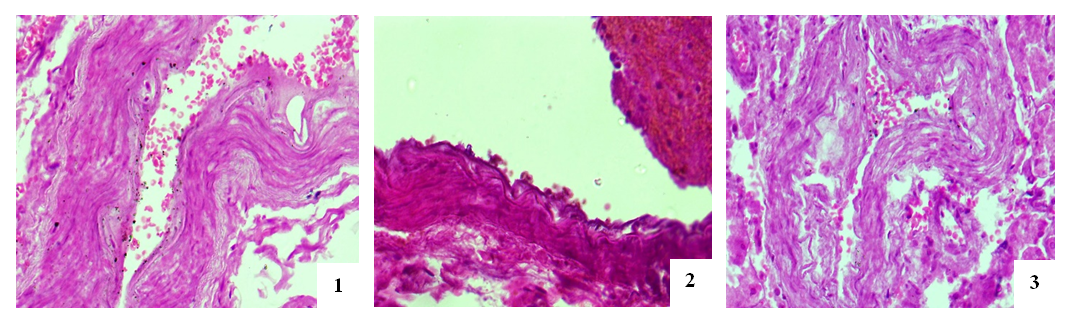
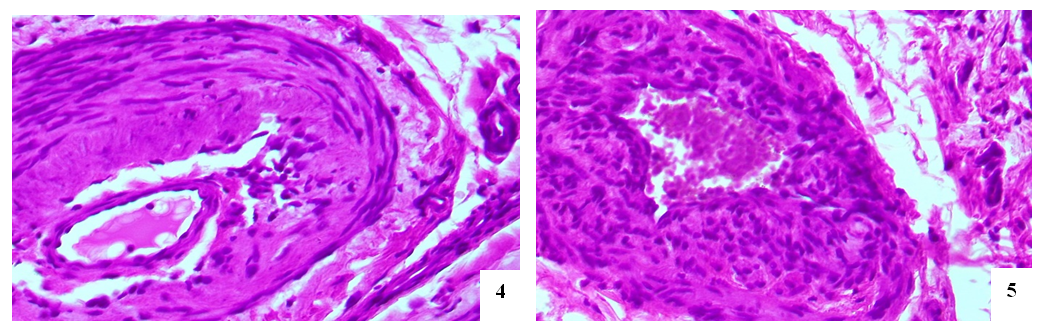
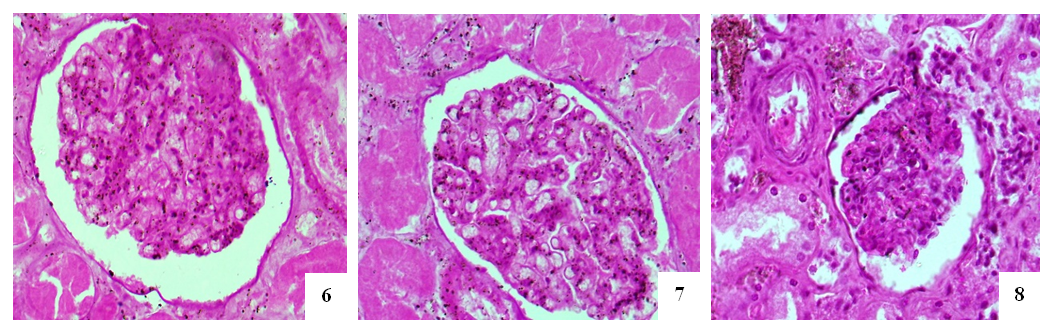
- The entrance artery of the kidneys, the arcuate artery between the cortical and medulla layers, the artery delivering blood to the capillaries and the efferent artery, including secondary peritubular branches of afferent and efferent arteries have been studied during morphological examination of the renal vessels. During studying the wall of the artery flowing into the kidneys, no significant pathomorphological changes in the outer adventitia, the middle muscle layer that forms the wall of this artery has not been found. Only in the intima of the inner surface of the artery wall, slight tortuosity, flattening of endotheliocytes, hyperchromization of the basal membrane, and deformation of its connective fibers have been revealed (Fig. 1). These changes were manifested by general pathomorphological changes that developed in response to the process of viral infection, i.e., general surface disorganization of tissue structures.While examining the wall of the arcuate arteries located between the layers of the cortical and medulla of the kidneys, it was noted that all layers of the arterial wall were equally affected by swelling and deformation. As a result, it was found that the cavity of the arteries have been narrowed, the erythrocytes in the cavity are located in a disorderly state, the wall of the artery is fused with the inner surface, and specific accumulations develop. It was noted that the intimate layer of the arterial wall was more edematous, thickened, bulged in some areas, and sank into the muscle layer in other areas. Plasma proteins were found to be concentrated on the inner surface of the intima layer, forming a homogeneous protein with a pale eosinophilic appearance (Figure 2). Endothelial cells were stretched and deformed due to swelling and disorganization. It was revealed that the basal membrane was some degree thickened and partially deformed due to edema and plasmarrhagia. The appearance of macrophages and lymphocytes in some areas of the intima of the arterial wall was noted. When studying the muscle layer, it was found that muscle cells and myofibrils were located in the same direction, fragmented and vacuolated due to the presence of interstitial swelling in some places. Strong pathomorphological changes due to edema and disorganization in the adventitial layer, as well as in the intima, were revealed. It was noted that the vessels in the adventitia were full–blooded, blood was poured out around them by diapedesis method, the fibrous structures of the connective tissue were homogenized due to mucoid swelling, and were destroyed in places. It was revealed that the relatively smaller network of arcuate arteries between the layers of the kidney has also been greatly deformed; edema, mucoid swelling, and fibrinoid necrosis have been developed in its parietal layers. It turned out that the cavity of this artery was also significantly narrowed and the erythrocytes were located without any order.It was revealed that some of the endothelial cells of the inner layer were swollen, some were displaced and desquamated, and lymphocytes were attached to some of them. Some areas of destruction in the muscle layer of the walls of these arteries have been observed; they have turned into a structureless tissue due to myolysis, myorhexis and destruction of muscle cells (Fig. 3). Areas of fibrinoid necrosis, hemorrhages, and severe edematous foci have been found in the interstitial connective tissue around the artery.
5. Conclusions
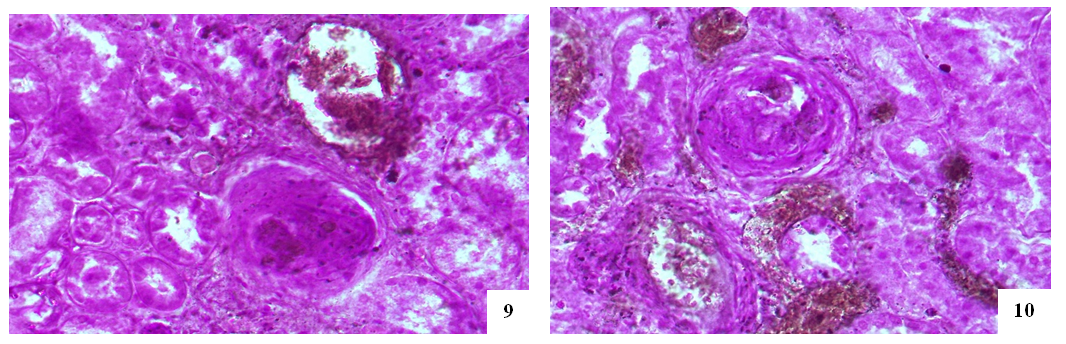
- In case of COVID–19 disease, minor arcuate artery between the cortical and medulla layers was damaged in greater degree than the major arteries of the kidney, all layers of the walls of these arteries were diffusely swollen and disorganized, desquamation of the endothelium in the intima showed adhesion of plasma proteins and erythrocytes.Various changes develop in the afferent and efferent kidney arteries demonstrating thickening of the intima cells in the wall of the afferent artery due to hypertrophy of the intima cells and the development of lymphohistiocytic infiltrate, endothelial proliferation in the wall of the efferent artery, thickening of the muscle layer and disruption of the location in the it muscle cells have been observed.It has been established that in some cases the microvessels of the capillary network of renal glomeruli are significantly dilated and blood-filled, in others they are spasmodic, the basal membrane of the capillaries is thickened and mucoid, the cells of the endothelium, podocytes and mesangium are proliferatively activated, the capillary network in one case was dilated and increased, and in another collapsed and wrinkled.It was noted that the peritubular artery in the interstitium of the cortical layer of the kidney proved to be more damaged, an obstructive thrombus developed in its cavity, several fragments of a thrombus of different sizes appeared in one vessel, the veins around it were well–filled.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML