-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 74-80
doi:10.5923/j.ajmms.20231302.10
Received: Dec. 13, 2022; Accepted: Feb. 6, 2023; Published: Feb. 13, 2023

Technology of Preparation and Evaluation of Properties of Brucellosis Hyperimmune Rabbit Serum
Bektimirov M. Amir, Tadjieva U. Nigora, Kasimov Sh. Odiljon, Yusupov P. Akmal
The Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious Diseases and Parasitic Diseases Tashkent, Uzbekistan
Correspondence to: Yusupov P. Akmal, The Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious Diseases and Parasitic Diseases Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A bank of rabbit hyper-immune anti-brucellosis sera was created (72 sample variants), including: serum before immunization, after 1, 2, 3, 4 immunizations, 13 days after the last 4th immunization; options for immunization with live attenuated brucellosis vaccine and inactivated culture; without adjuvant and with incomplete Freund's adjuvant.
Keywords: Brucellosis, Brucella abortus 19, Hyperimmunization, Standard serum, Albumin, Globulin, IgA, IgM, IgG, Heddelson reaction, Wright reaction
Cite this paper: Bektimirov M. Amir, Tadjieva U. Nigora, Kasimov Sh. Odiljon, Yusupov P. Akmal, Technology of Preparation and Evaluation of Properties of Brucellosis Hyperimmune Rabbit Serum, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 74-80. doi: 10.5923/j.ajmms.20231302.10.
1. Introduction
- Along with the increase in the number of cases of infectious diseases among the world's population, the number of zoonotic diseases, including brucellosis, is also growing daily. One of the main reasons is the consumption of milk and meat of animals with brucellosis [10].According to the WHO Joint Committee on Brucellosis, brucellosis has been registered among animals in 155 countries around the world. Brucellosis is most widely spread in the countries of South and Southeast Asia, Africa, Central and South America. Every year, more than 500,000 cases of this disease are registered in the world of people suffering from its negative consequences [7].In order to effectively control the activity of epizootic and epidemic factors in areas endemic to brucellosis, the use of diagnostic laboratory examination methods for this nosology is carried out starting from the patient's admission to the primary health care unit [1,5]. This is due to the variety of clinical signs of brucellosis, the similarity of its clinical course with other diseases, the absence of strictly specific symptoms, which often causes difficulty in making a preliminary diagnosis of "Brucellosis", leading to an erroneous diagnosis with incorrect therapy. Often such situations arise with incomplete collection of anamneses, the absence of cardinal symptoms of the disease, with super- and reinfection, the development of relapses and latent forms of infection. In such cases, even in non-endemic areas for brucellosis, laboratory research methods become important [11].Today, one of the reliable, auxiliary methods in the diagnosis of brucellosis, both in humans and in animals, is the conduct of serological tests. Of the serological methods of research in humans, reactions are currently used: Heddelson agglutination (one of the methods of rapid diagnosis of brucellosis), Wright, passive hemagglutination, Coombs reaction (indirect antiglobulin test), etc. At the same time, the Heddelson reaction is the most sensitive reaction than the Wright reaction, but less specific. Negative results of Heddelson's reaction indicate the absence of brucellosis activity, but positive ones do not always reliably confirm the diagnosis of the disease. The Wright agglutination reaction is a highly specific reaction, with titers of 1:50 and above usually confirming the diagnosis of brucellosis. In animals from serological reactions, the following are used: The Rose Bengal test (RBT), the agglutination and complement binding reaction [2,4,6]. In order to standardize and improve the effectiveness of the above methods, in accordance with the requirements and recommendations of the FAO/WHO Association of Experts on Biological Standardization, each country should use its own individual national standard serum with the appropriate titer, which is expressed in international units (IU/ml) [3,8].The absence of national standard serums causes problems in quality control of commercial and local antigens, hinders the development of local drugs - brucellosis diagnostics, which reduces the effectiveness of serological diagnostics of brucellosis in humans and animals [9].Considering the above, it becomes necessary to develop and produce a standard anti-brucellosis serum, check these products for quality.
2. Materials and Methods
- To obtain positive standard serums against brucellosis pathogens, 12 rabbits weighing from 2.2 kg to 4.6 kg aged 6.0 to 12 months were used. The animals were kept in the same conditions on a standard vivarium diet. For the experiment, animals adapted to the conditions of the experiment were taken, which were kept in quarantine for 21 days. To conduct the study, biomodels were anesthetized according to the international rules for the humane treatment of animals (Order No. 755, 1977; Order No. 742, 1984). The rabbits were divided into 4 groups. Each rabbit from group 1 was immunized 4 times by introducing a weakened live B. abortus 19 vaccine with a virulence concentration corresponding to 4 billion. In the 2nd group of rabbits, the 2nd and 3rd immunization was also carried out by administration of a weakened live B. abortus 19 vaccine with a virulence concentration corresponding to the 4th billion, and the 1st and 4th immunization was carried out by administration of a live B. abortus 19 vaccine with an incomplete Freund adjuvant with a virulence concentration corresponding to the 4th billion (an incomplete Freund adjuvant was prepared by adding two ml of BCG vaccine to 6 ml of Vaseline oil).Each rabbit from the 3rd group was injected with a suspension of inactivated brucella culture at a concentration of 1 billion (according to the McFarland standard) 2 times, the 3rd immunization was carried out with a concentration of 4 billion, the 4th immunization was carried out with a live B. abortus 19 vaccine with a virulence concentration corresponding to 4 billion.Each rabbit from the 4th group during the 1st immunization was injected with a suspension of inactivated brucella culture at a concentration of 1 billion together with an incomplete Freund adjuvant, the 2nd immunization was carried out by introducing a suspension of inactivated brucella culture at a concentration of 1 billion, the 3rd immunization - at a concentration of 4 billion, and at the 4th immunization was administered a live B. abortus 19 vaccine with a virulence corresponding to 4-mm billion.All rabbits were injected with antigen (live vaccine or suspension of inactivated brucella culture) at a total of 8 points (1-4 points - subcutaneous injection, 5-8 points - intramuscularly) between the muscles.Bacteriological method. In the process of obtaining serums, we used strains of B.abortus 19 vaccines with the lowest virulence. The vaccine was produced in the Shchelkovsky Biocombinat of the Russian Federation, serial number 204, production time - April 2022, expiration date - 12 months. The introduction of the B.abortus 19 vaccine was carried out in experimental rabbits by pre-growing an inactivated brucella suspension, according to the McFarland standard, diluted in saline solution.A feature of the serums was the study of clinical strains of Enterobacteriaceae spp. 74, Proteus vulgaris 86, Salmonella spp.632, Salmonella spp. 660, Salmonella spp. 662, Salmonella spp. 663, Salmonella spp. 760, Salmonella spp. 766, Klebsiella pneumoniae 97, Salmonella spp. 696, Shigella spp. 5, E. coli 108, E. coli 109, E. coli 110, E. coli 111, E. coli 112, E. coli 114, E. coli 914, Klebsiella pneumoniae 128, Citrobacter spp. 134 Klebsiella pneumoniae 139, Citrobacter spp. 140, Shigella spp. 973, Shigella spp. 1128 isolated in the bacteriological laboratory from patients with acute diarrhea and acute intestinal infection who received inpatient treatment at the clinic on the basis of the Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases.Serological method: was carried out on the basis of Appendix 12 (methodological instructions for laboratory diagnosis of brucellosis) of the Order of the Minister of Health No. 177 dated May 1, 2015 "On improving laboratory methods conducted in laboratories of bacteriological, virological and especially dangerous infectious diseases". Immunological studies. Identification of immunoglobulins of classes A, M and G against brucellosis pathogens by the enzyme immunoassay was carried out using a set of reagents Vector BEST, RF. Determination of the level of total protein, albumin and globulin was established by the enzymatic colorimetric method by using the biochemical analyzer "Mindray" ВA-88A on medical equipment of a Chinese company. The results were evaluated based on the manufacturer's instructions.Statistical method: digital material was processed by the method of variational statistics using the program "Excel-Office" 2013 using the Student's t-test. The mean quadratic error (m) was calculated, as well as the reliability of the differences in the values in the compared groups. The differences were considered significant at p<0.05. Nominal data are described with absolute values and percentages. The nominal data were compared using Pearson's χ2 criterion, Fisher's exact criterion. The differences were considered significant at p<0.05.
3. Results and Discussion
- Before hyperimmunization of experimental rabbits, their blood serum was subject to serological (using the Wright-Heddelson reaction) and immunological examination for brucellosis (identification of IgA, IgM and IgG by the enzyme immunoassay). In addition, the level of total protein, albumin, globulin and the ratio of albumin to globulin were determined in rabbits.Experimental animals were hyperimmunized 4 times with an interval of 7 days. The day before each hyperimmunization, the blood serum of experimental animals was examined using the above-mentioned serological and immunological reactions. 13 days after the last 4th hyperimmunization, the experimental rabbits underwent total blood sampling. In accordance with the "veterinary and sanitary rules for the collection, disposal and neutralization of biological waste", approved by Resolution No. 13, 12 (100%) of the State Committee for the Development of Veterinary Medicine and Animal Husbandry dated October 14, 2019, rabbits used in the experiment were destroyed by burning at a high temperature of 700oC in a special crematorium furnace located in scientific center of Pharmaco-toxicology and vivarium. The blood serum from each experimental animal was poured into separate sterile jars with the addition of the highest concentration sodium merthiolate as a preservative in a ratio of 1:10,000, followed by heating the serum in a water bath at 56°C for 30 minutes, stirring constantly. In order to conduct serological and immunological studies, one part of the serum was placed in refrigerators with a temperature of 2-8°C, and the second part was stored in freezers at a temperature of minus 20°C for subsequent use. Evaluation of serum specificity in heterologous microorganisms. The serum obtained as a result of hyperimmunization may react with agglutination, intersecting with antigens of pathogens of other infectious diseases, which in turn may cause erroneous identification of the isolated strain. Therefore, it is important to identify the features and specificity of the serum, check it for the presence of other pathogens of the disease.Therefore, the specificity of the sera obtained as a result of the experiment was studied using clinical strains of gram-negative bacteria isolated from patients undergoing treatment with diagnoses of acute diarrhea or acute intestinal infection in the bacteriological laboratory of the clinic located on the basis of the Republican Specialized Scientific and Practical Medical Center for Epidemiology, Microbiology, Infectious and Parasitic Diseases. Thus, the blood serum obtained from experimental rabbits in different periods of the study: before pre-immunization, during hyperimmunization, as well as during total blood collection, was subjected to an agglutination reaction on a slide with all clinical strains. As a result of the agglutination reaction with gram-negative bacteria (Citrobacter spp. Enterobacteriaceae spp.), a weakly positive response (+) was established on a slide in 16.7% of cases. In a further study, it was these blood serums that were subjected to an agglutination reaction in test tubes with the addition of a suspension of inactivated the same microorganisms (Citrobacter spp. Enterobacteriaceae spp.). The results were negative. The results of the study of sera after hyperimmunization are presented in Tables 1-4. Table 1 provides information on the results of immunization of experimental animals of the first group.
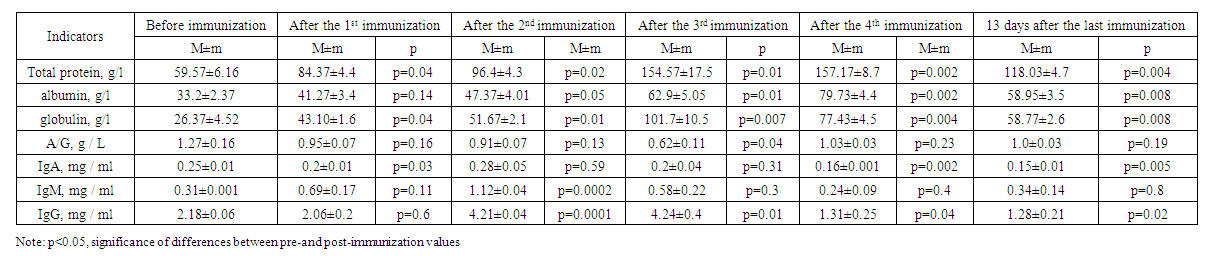 | Table 1. Results of immunization of experimental animals of the first group |
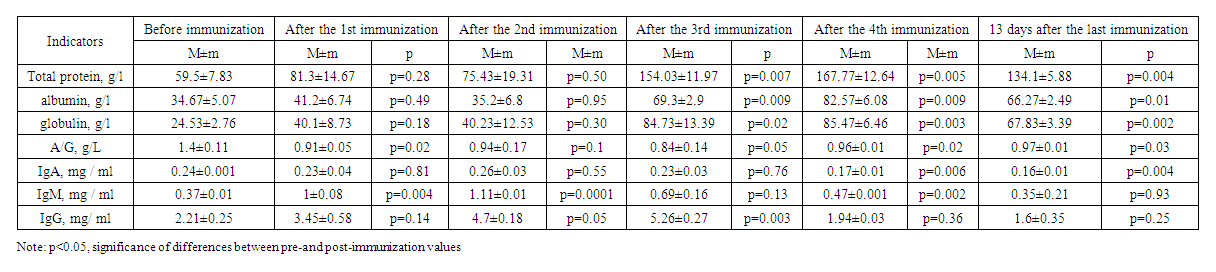 | Table 2. Results of immunization of experimental animals of the second group |
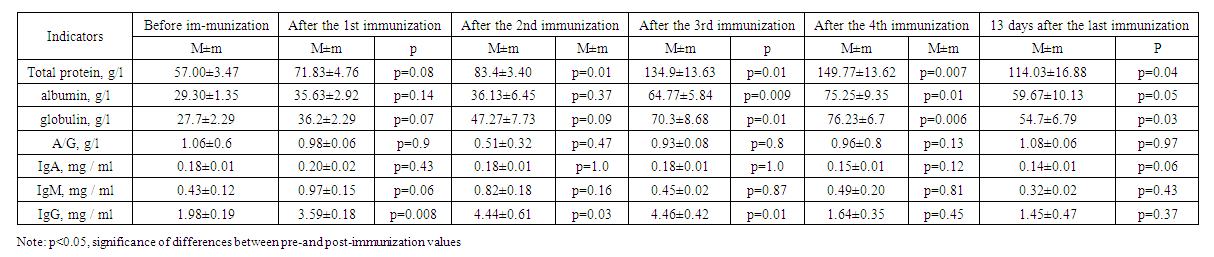 | Table 3. Results of immunization of experimental animals of the third group |
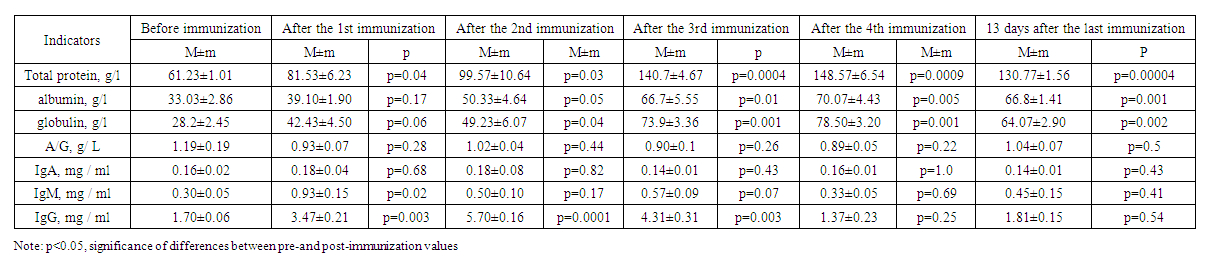 | Table 4. Results of immunization of experimental animals of the fourth group |
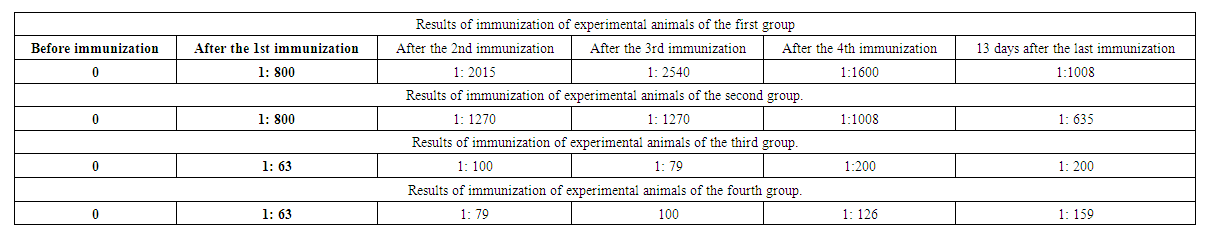 | Table 5. Results of studies on the geometric mean titer of the Wright reaction |
4. Conclusions
- 1. In the process of repeated immunization, an increase in the level of total protein, albumin, globulin, IgM and IgG indicates the formation of immunity to antigens (an increase in IgM levels in the first week of primary immunization indicates the formation of primary immunity, an increase in IgG levels from the second week indicates the formation of secondary immunity).2. The use of an incomplete Freund adjuvant leads to the formation of a stable immune response.3. Administration of the B. abortus 19 vaccine strain and suspension of a weakened brucella culture showed the development of a more stable and long-lasting immune response (during the observation period).4. The titer of antibodies in experimental animals of groups 3 and 4, immunized with a suspension of inactivated brucella culture, was at the same level in dynamics as in the first group, but was significantly reduced when immunized with a live vaccine B. abortus 19 with low virulence.5. To obtain a full-fledged, active hyperimmune vaccine serum, it becomes necessary to use a live, weakened bacterium.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML