-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 67-70
doi:10.5923/j.ajmms.20231302.08
Received: Jan. 9, 2023; Accepted: Feb. 1, 2023; Published: Feb. 13, 2023

Mechanism of Sclerosis and Pathomorphology of the Stroma of the Middle Lobes of the Prostate Gland
Xamraev O. A.
Andijan State Medical Institute, Andijan, Uzbekistan
Correspondence to: Xamraev O. A., Andijan State Medical Institute, Andijan, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, pathomorphological changes were studied that develop as a result of chronic inflammation in the interstitial tissue of the glandular region around the urethra from various topographic regions of the prostate gland. At autopsy of the RPAC, the middle lobe of the prostate gland was removed from the corpses of men and examined histologically. The results obtained show that the development of fibromatosis in the periurethral region of the prostate gland is characterized by pathomorphological changes characteristic of long-term chronic inflammation, i.e., first the appearance of a lymphohistiocytic infiltrate, then the proliferation of connective tissue occurs with an increase in the proliferative activity of histiocytic cells. At the same time, differentiated fibroblasts, underdeveloped fibroblasts, differentiated fibroblasts and myofibrocytes multiply and turn into fibroblasts, which synthesize a large amount of collagen and form fibrous tissue. In some cases, myxomatosis of fibromatous tissue is due to a large accumulation of acidic glycamingglycans from 2 types of polysaccharides in the intermediate substance, the acidic environment and hydrophilicity of the tissue increase, the acidic dye eosin is less absorbed, and basophilic staining is observed.
Keywords: Prostate, Middle lobe, Prostatitis, Sclerosis, Fibrosis, Morphogenesis, Pathomorphology
Cite this paper: Xamraev O. A., Mechanism of Sclerosis and Pathomorphology of the Stroma of the Middle Lobes of the Prostate Gland, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 67-70. doi: 10.5923/j.ajmms.20231302.08.
Article Outline
1. Introduction
- Sclerosis of the prostate gland is considered a progressive process due to inflammation, and the stroma of the organ becomes denser due to the accumulation of collagen. On average, 13% of 40-50-year-old men develop prostate sclerosis due to chronic inflammation [1,2,3]. Benign hyperplasia of the gland due to the development of sclerosis in the prostate is characteristic. In most men, due to this disease, obstruction of the urinary tract, that is, narrowing of the urethra, and sexual disorders are observed. The causes are infection, autoimmune process and chronic inflammation that develops as a result of allergies [1,6,8]. The following can be indicated as risk factors: blood circulation and metabolism disorders due to diffuse atherosclerosis, aging of men, hypoandrogen status, genetic predisposition, connective tissue diseases, autoimmune diseases, chronic poisoning, neurofibromatosis and cystic fibrosis diseases [1,2,5]. As a pathogenesis of the disease, it can be shown that as a result of chronic inflammation, paracrine signals appear in lymphocytes and macrophages from the cells of the immune system, which transform myofibroblasts in the prostate stroma into proliferative fibroblasts. In addition, collagen formation is enhanced with the help of cytokines, chemokines and growth factors. Instead of the end of the inflammatory process with normal regeneration, reparation develops and the mechanism of the formation of excess connective tissue remains unknown [4,7,8]. The diagnosis of prostate sclerosis is made by histological examination, which is the golden rule in urology, and it can show several types: focal changes, nodular adenomatous hyperplasia, sclerosis and atrophy of the parenchyma, and sclerosis with cystic changes [1,9].The prostate is a muscular-glandular organ that surrounds the upper part of the urethra. The outer surface of the gland is surrounded by a connective tissue membrane. Parenchyma consists of a large number of mucous glands, excretory ducts adjacent to the urinary tract. Glands are located around the urethra in three groups: central, peripheral and intermediate glands. The prostate is supplied with blood from the lower arteries of the bladder and the middle artery of the rectum. Central glands are located under the mucous membrane of the urethra, intermediate glands are located between the connective tissue of the prostate gland, and peripheral glands are located in the greater part of the prostate gland. The main goal of this study is to study the pathomorphological changes that develop due to chronic inflammation in the interstitial tissue of the glandular area around the urethra from different topographical areas of the prostate gland.
2. Material and Methods
- Men's prostate gland as an object of examination Prostate gland was isolated during autopsy of men who died of cardiovascular, respiratory and gastrointestinal diseases in the Department of Adult Pathology of the Republican Center for Pathological Anatomy. The periurethral glandular area forming the wall of the urethra of the gland, i.e., the middle part, was topographically separated and divided into pieces and frozen in 10% formalin solution for 48 hours for histological examination. After washing in running water for 4 hours, they were dehydrated in increasing concentrations of alcohols and embedded in paraffin, and sections were prepared. 4-5 μm histological sections were prepared from paraffin blocks and stained with hematoxylin-eosin and van Gieson methods. Preparations were viewed and studied under a light microscope, and necessary areas were photographed.
3. Research Results and Discussion
- The interstitium of the prostate gland consists of unformed connective tissue and myofibroblast, smooth muscle cells. In the normal state, the interstitial tissue of the prostate contains poorly developed fibroblasts, differentiated fibroblasts, fibroblasts, and contractile myofibroblasts. In addition, there are histiocytes and plasmocytes, lymphocytes, mast cells, adventitial cells, and pericytes that perform the function of macrophages. Fibrous structures include collagen, elastic and reticular fibers. The composition of the intermediate substance consists of a homogeneous, amorphous, gel-like main substance consisting of polysaccharides and tissue fluid. Polysaccharides occur in two forms: sulfated and non-sulfated glycosaminoglycans. The peculiarity of the interstitium of the prostate gland is that there is a myxomatous tissue rich in hyaluronic acid, which provides tissue turgor. In order to achieve the goal, the following data were obtained by looking at the histological preparations prepared to study the pathomorphological changes that develop due to chronic inflammation in the glandular area around the urethra of the prostate gland, i.e. the interstitial tissue of the middle part of the gland. Since this area of the prostate gland is located close to the urethra, it was found that the inflammatory process occurs as a result of infectious processes developed in the urethra. In fact, it is determined that the relatively small gland structures in the middle part of the prostate around the urethra are hyperplastic in response to the inflammatory process, the gland structures are of different sizes, and the gland epithelium in them is hyperchromic. As pathomorphological changes characteristic of chronic inflammation, infiltration of lymphoid and histiocytic cells in the connective tissue interstitium around glandular structures is determined. In lympho-histiocytic infiltration, lymphocyte and plasma cells from immune system cells, histiocyte and histioblast cells from local histiocytic cells have increased, some of them have become macrophages. In addition, differentiated fibroblasts, poorly developed fibroblasts, differentiated fibroblasts, and myofibrocytes have increased from the cells of the fibroblast line. From these, it is determined that differentiated, immature fibroblasts and histioblasts gathered in one place and formed an infiltration (Fig. 1), myofibroblasts and smooth muscle cells proliferated and multiplied, forming muscle tissue tufts of various sizes around and between the glands.
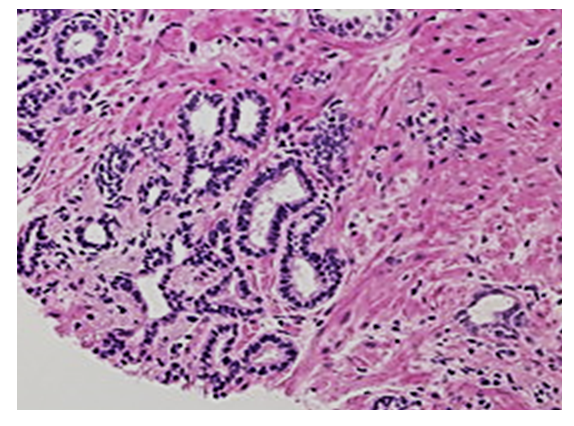 | Figure 1. The middle part of the prostate gland around the urethra, small glandular structures are hyperplastic, lympho-histiocytic cells have infiltrated around it. Paint: G-E. Magnified: 10x40 |
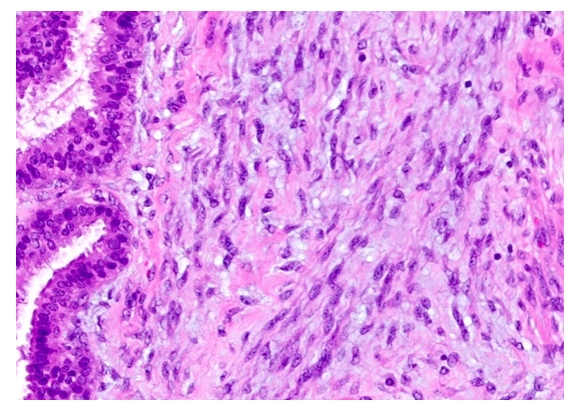 | Figure 2. Proliferation of histiocytic cells in the middle part of the prostate gland around the urethra, interstitial myxomatosis. Paint: G-E. Magnified: 10x40 |
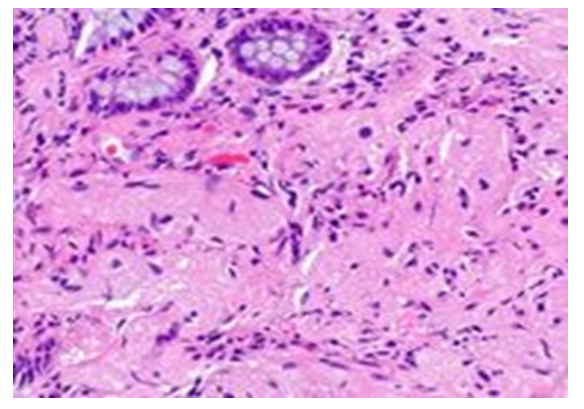 | Figure 3. Fibromatous tissue appeared in the stroma of the middle part of the prostate gland around the urethra. Paint: G-E. Magnified: 10x40 |
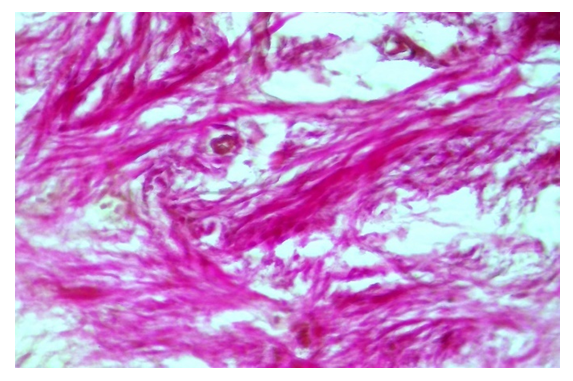 | Figure 4. In the middle part of the prostate gland around the urethra, fibromatous tissue grows and collagen fibers increase. Paint: van Gieson. Magnified: 10x40 |
4. Conclusions
- The development of fibromatosis in the periurethral area around the urethra of the prostate gland is characterized by pathomorphological changes characteristic of long-term chronic inflammation, i.e. the appearance of a lympho-histiocytic infiltrate, then the growth of connective tissue with increased proliferative activity of histiocytic cells. In this case, differentiated fibroblasts, poorly developed fibroblasts, differentiated fibroblasts and myofibrocytes from the cells of the fibroblast line multiply and turn into fibroblasts, which synthesize a large amount of collagen and form fibrous tissue. In some cases, myxomatosis of fibromatous tissue is caused by the large accumulation of acidic glycaminglycans from 2 types of polysaccharides in the intermediate substance, the acidic environment and hydrophilicity of the tissue increases, the acidic dye eosin is less absorbed, and basophilic staining is observed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML