-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(2): 51-56
doi:10.5923/j.ajmms.20231302.04
Received: Jan. 15, 2023; Accepted: Feb. 1, 2023; Published: Feb. 13, 2023

Markers of Ovarian Reserve in Women with Premature Ovarian Insufficiency Depending on the Level of Follicle-Stimulating Hormone
Fakhrutdinova Sevara
Republican Specialized Scientific and Practical Medical Center for Endocrinology Named after Academician Y.Kh. Turakulova, Tashkent, Republic of Uzbekistan
Correspondence to: Fakhrutdinova Sevara, Republican Specialized Scientific and Practical Medical Center for Endocrinology Named after Academician Y.Kh. Turakulova, Tashkent, Republic of Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

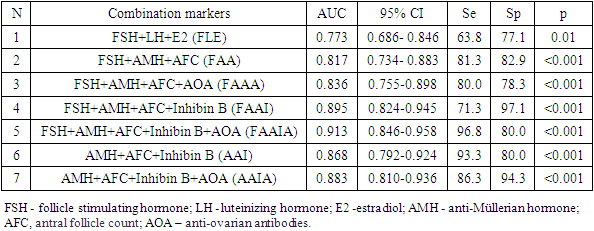
Premature ovarian insufficiency (POI) is a condition characterized by non-reversible decreased ovarian function outside the normal range for women under 40 years of age. Purpose of the Study: The purpose of the study is to evaluate the indicators of ovarian reserve in women with POI with different levels of FSH. Materials and Methods: The main group included 80 women (average age 28.0±7.2 years) with an idiopathic form of POI. The control group included 35 women (average age 30.6±8.3 years) with regular menstruation and without ascertained endocrine disorders. Results: All women with POI were divided into 3 groups: group 1- high-risk of POI (pre-POI) - FSH <25 mIU/ml (n=15), group 2- early POI - FSH 25-40 mIU/ml (n=16) and group 3 -POI FSH ≥40 mIU/mL (n=49). It should be noted that the majority (61.3%) of women with POI had FSH levels ≥40 mIU/ml. Diagnostically significant cut-off points were determined for AMH (1.08 ng/ml), inhibin B (2.85 pg/ml), AOA (2.26 U/ml), and AFC (4). The highest percentage of correct predictions (93.8%) of POI development is observed in the case of using a combination of such markers as FSH + AMH + AFC+ Inhibin B + AOA (AUC-0.913; 95% CI 0.846-0.958).
Keywords: Premature ovarian insufficiency (POI), Hormonal profile, Ovarian reserve
Cite this paper: Fakhrutdinova Sevara, Markers of Ovarian Reserve in Women with Premature Ovarian Insufficiency Depending on the Level of Follicle-Stimulating Hormone, American Journal of Medicine and Medical Sciences, Vol. 13 No. 2, 2023, pp. 51-56. doi: 10.5923/j.ajmms.20231302.04.
Article Outline
1. Introduction
- Premature ovarian insufficiency (POI) is a condition characterized by non-reversible decreased ovarian function outside the normal range for women under 40 years of age [1].According to a number of researchers, POI exists as a continuum of changes in ovarian function, which includes a "latent" condition in which women have reduced fertility but normal FSH levels and regular menstruation, a "bio-chemical" state with reduced fertility, elevated FSH levels, but regular menstruation and finally "explicit" condition with reduced fertility, elevated FSH levels, and irregular or no menstruation. [2,3,4,5].In clinical practice, the state of ovarian reserve (OR) is assessed using elevated FSH levels, low levels of AMH and E2, decrease in the number of antral follicles (AFC). There is no optimal test for assessing OR, and the data of tests for OR are often very contradictory.Anti-Mullerian hormone (AMH) and the number of antral follicles (AFC) are indispensable markers of the follicle pool and ovarian reserve. Both low AMH and low AFC may contribute to determining the age of early menopause. [6].According to various authors, 5th percentile of the age level of AMH corresponds to women who have undergone relatively early menopause. In 35% of cases, AFC ≤4 indicates an increased risk of menopause within 7 years compared with the risk in women with AFC >4 (13%) [7,8].Lunding S. et al. [9] note that AMH threshold value of the hormone corresponding to 3 pmol / l is a predictor of imminent POI in women, sensitivity and specificity is 95%. Results of the study conducted by Khaidarova F.A. [10] indicate the existence of correlation between the level of AMH and AFC, and are markers of ovarian aging.Wen J. et al. [11] studied the relationship between inhibin B and classical markers of ovarian reserve and function. The results of the study showed that the levels of inhibin B decreased significantly in women when they are older than 40. Inhibin B was positively correlated with AMH (r= 0.57; p<0.001), AFC (r = 0.34; p<0.001) and testosterone (r = 0.10; p = 0.002) and negatively with FSH (r = -0.41; p<0.001) and LH (r = -0.20; p<0.001) and FSH / LH (r = -0.18; p<0.001), while no correlation with PRL was detected. Prognostic significance of inhibin B (AUC = 0.74; p<0.001 for the population of studied institutions; AUC=0,78; p<0.001 for the validation population) was higher than FSH (AUC=0.71; p<0.001 for the population of institutions studied; AUC=0,72; p<0.001 for the validation population) in the diagnosis of AFC<5-7 [11].Welt C. et al. [12] have found that decrease in the level of inhibin B is an early marker of decrease in the number of follicles in the process of reproductive aging. The authors state that inhibin B is one of the prognostic factors in the restoration of ovarian function in patients with POI.
2. Purpose of the Study
- The purpose of the study is to evaluate the indicators of ovarian reserve in women with POI with different levels of FSH.
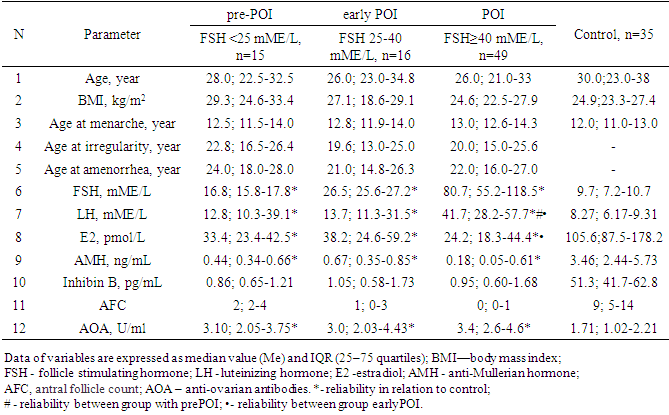
3. Materials and Methods
- The main group included 80 women (average age 28.0±7.2 years) with an idiopathic form of POI. The control group included 35 women (average age 30.6±8.3 years) with regular menstruation and no confirmed endocrine disorders. All women in the study signed an informed consent to an anonymous analysis of their medical data. According to ESHRE recommendations, the diagnostic criteria for POI are: oligo / amenorrhea for at least 4 months and elevated FSH levels >25 IU / l in two cases with an interval of >4 weeks [13].Blood sampling was performed in the follicular phase (3-5th day of the menstrual cycle) in controls and against the background of amenorrhea in women with suspected POI. Serum hormone levels were determined (FSH, LH, E2 (estradiol), AMH, inhibin B) by electrochemiluminescent method on Elecsys immunochemical analyzer and cobas e. using standard Cobas Roche kits («Roche Diagnostics GmbH», Germany). Anti-ovarian antibodies (AOA) in blood serum were determined by enzyme immunoassay using kits by "Human" company (Germany).The normal reference ranges used in our laboratory were as follows: FSH: 3.5–12.5 mIU/ml; LH: 2.4–12.6 mIU/ml; E2: 68–1269 pmol/L; AMH: 0.09-9.49 ng/ml; inhibin B: from 0 -273 pg/ml; АОА: 0-10 U/ml.To assess the ovarian reserve (OR), the volume of the ovaries and the number of antral follicles (AF) in each ovary were determined on the 5th-7th day of the menstrual cycle on DC-40 Mindray ultrasound scanner (South Korea) using V10-4 transvaginal sensor with frequency 4 -9 MHz.
4. Statistical Analysis
- Statistical processing of the results was carried out using Microsoft Excel, IBM SPSS Statistica 23 and MedCalc version 18.5. The initial data were evaluated for compliance with the normal distribution according to Kolmogorov-Smirnov criterion.Dependences were analyzed using Spearman's rank correlation coefficients. To assess the prognostic significance of markers, ROC analysis (Receiver Operating Characteristic) was used, with the calculation of the area under curve (AUC), Se (sensitivity) and Sp (specificity) of the model. Results are presented as median (Me) [interquartile range Q25; Q75]. Differences were considered statistically significant at р<0,05.
5. Results and Discussion
- All women with POI were divided into groups based on ESHRE recommendations, FSH group >25 to 40 mIU/mL included 16 women, FSH group ≥40 mIU/mL included 49 patients.Since the maximum reference range of FSH in our laboratory was 12.5 mIU/ ml, the study included women under 40 years of age with menstrual irregularities (secondary amenorrhea lasting 6 months or more), with FSH levels of <25 mIU / ml, high LH levels and low E2 obtained twice with an interval of 4 weeks, the concentration of AMH was also taken into account. We designated this cohort of women (n = 15), as a high-risk group of POI (average age 28.7±8.0 years), they did not take medications (for 6 months before the examination), which could have an impact on hormonal and biochemical indicators.Thus, 3 groups of patients were identified: group 1 -at high risk of POI (pre-POI) - FSH <25 mIU/ml (n=15), group 2- early POI - FSH 25-40 mIU/ml (n=16) and 3 POI group- FSH ≥40 mIU/ml (n=49). It should be noted that the majority (61.3%) of women with POI had FSH levels ≥40 mIU/ml.The analysis revealed no differences in age and BMI between the three groups. The age of the examined patients and persons from the control group ranged from 18 to 45 years. (Table 1.).
|
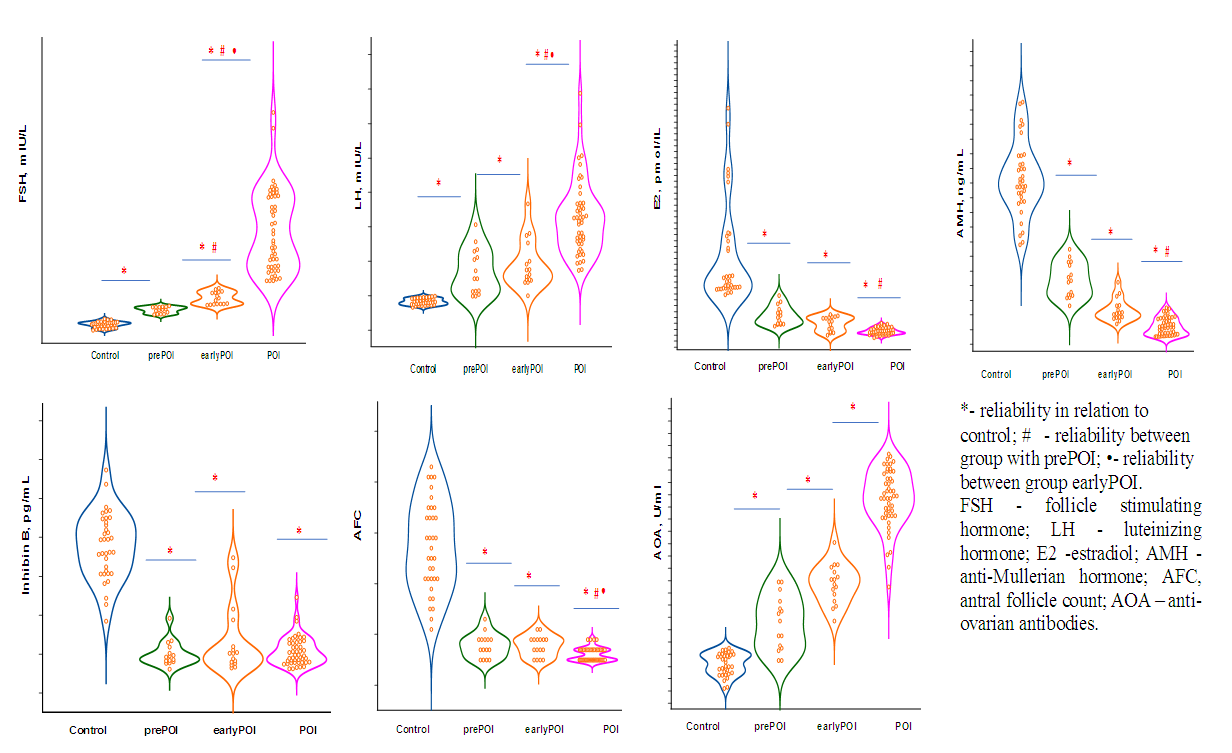 | Figure 1. Change in markers of ovarian reserve at various stages of ovarian insufficiency |
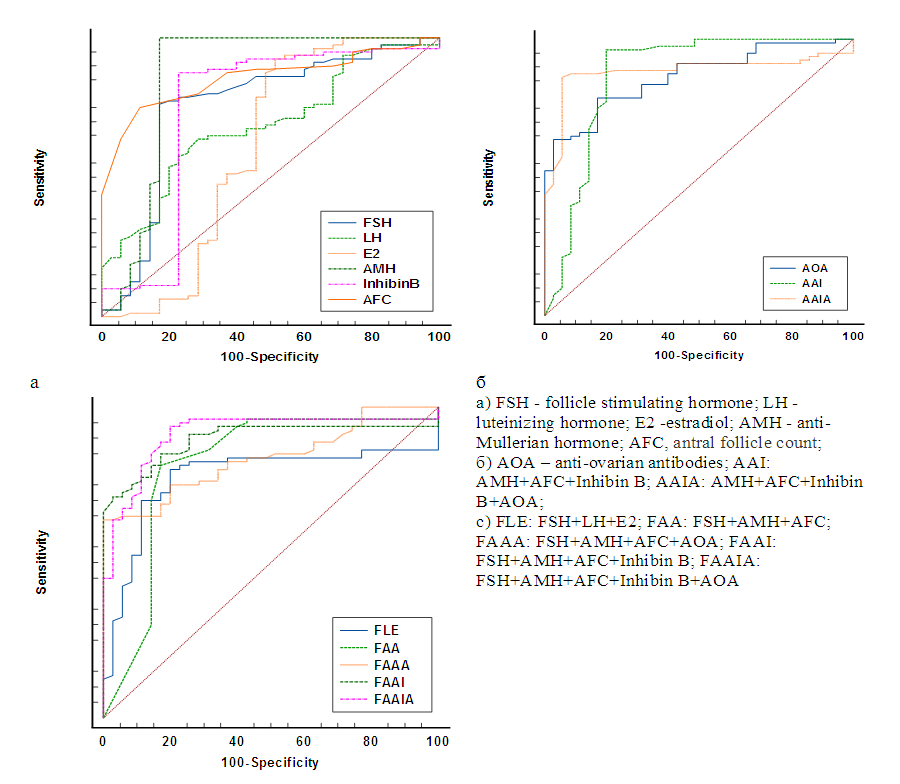 | Figure 2. ROC curves for POI prediction |
|
6. Conclusions
- 1. With the help of ROC analysis, diagnostically significant cut-off point for AMH (1.08 ng / ml), inhibin B (2.85 pg / ml), AOA (2.26 U / ml) and AFC (4) are determined, the values of these markers are the starting point for identifying the risk group of POI.2. Inclusion of AMH and AFC in FSH model significantly improved its predictive power (AUC-0.817; 95% CI 0,734-0,883).3. The highest percentage of correct predictions (93.8%) of POI development is observed when using a combination of such markers as FSH + AMG + AFC + Inhibin B + AOA (AUC-0.913; 95% CI 0,846-0,958).
Conflict of Interest
- The author has no conflict of interest to declare.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML