-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(12): 1334-1337
doi:10.5923/j.ajmms.20221212.32
Received: Dec. 7, 2022; Accepted: Dec. 28, 2022; Published: Dec. 30, 2022

Dexmedetomidine for Sedation in Patients with COVID-19 During Respiratory Support
Ibadov Ravshan Aliyevich1, 2, Ibragimov Sardor Khamdamovich1, Khakimov Begali Bobokulovich2
1Republican Specialized Scientific-Practical Medical Center of Surgery Named after Academician V.Vakhidov, Tashkent, Uzbekistan
2Republican Specialized Zangiota-2 Hospital for COVID-19, Tashkent Region, Uzbekistan
Correspondence to: Ibragimov Sardor Khamdamovich, Republican Specialized Scientific-Practical Medical Center of Surgery Named after Academician V.Vakhidov, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: to evaluate sedation therapy effectiveness in critically ill patients with severe COVID-19 who received dexmedetomidine compared to propofol. Methods. This was a prospective single center, cohort study of critically ill 333 adult patients with COVID-19 and psychoemotional disorders (depression, anxiety and posttraumatic stress disorder) admitted in the ICU of the Republican Specialized Zangiota Hospital for COVID-19. Patients were non-invasive ventilated more than 24 hours and received intravenous sedation with dexmedetomidine or propofol. Results. The risk of progression of the pathological process decreased from 47.6% to 21.8% and, accordingly, the proportion of patients with stabilization and improvement of their condition increased from 52.4% to 79.4% (χ2=26.979; p<0.001). The possibilities of non-invasive respiratory support were expanded with a reduction in the frequency of tracheal intubations from 17.3% to 7.3% (χ2=23.244; p<0.001), the duration of ICU stay - from 12.6±0.8 to 9.4±0.6 days (t=-2.89; p<0.05), and the duration of respiratory therapy - from 8.4±0.5 to 5.2±0.4 days (t=-5.20; p<0,05). In particular, there was an improvement in SpO2 recovery after one day of intensive therapy from 86.6±0.2% to 92.2±0.3% (t=7.64; p<0.05) with non-invasive ventilation and a higher oxygenation index (2.3 in the dexmedetomidine group versus 1.6 in the propofol group, p=0.032) during the period of sedation withdrawal. Conclusion. In the presence of severe psychoemotional disorders, the effectiveness of the application of protocols in the main areas of etiotropic and pathogenetic treatment of COVID-19 (correction of coagulopathy, respiratory and antibiotic therapy) directly depends on the proper sedation regimen. In this aspect, dexmedetomidine provides adequate and safe respiratory support with an improvement in external respiration, blood gas composition and a minimal negative hemodynamic effect.
Keywords: COVID-19, Depression, Anxiety, Posttraumatic stress disorder, Sedation, Dexmedetomidine, Comparison study
Cite this paper: Ibadov Ravshan Aliyevich, Ibragimov Sardor Khamdamovich, Khakimov Begali Bobokulovich, Dexmedetomidine for Sedation in Patients with COVID-19 During Respiratory Support, American Journal of Medicine and Medical Sciences, Vol. 12 No. 12, 2022, pp. 1334-1337. doi: 10.5923/j.ajmms.20221212.32.
1. Introduction
- Critically ill patients with COVID-19 are particularly at high risk of developing delirium, multiple organ failure, and, as a consequence, intensive care unit syndrome [1,2]. Thus, more than 80% of patients with COVID-19 receiving treatment in intensive care units have mental and neurological disorders, including sleep disturbances, headache, dizziness, myalgia, anxiety and depression, delirium/encephalopathy, psychomotor agitation, stroke, ischemic brain damage, convulsions, coma and meningoencephalitis [2,3]. According to preliminary results of retrospective cohort studies, neurological manifestations of varying severity are often observed even without symptoms of respiratory failure [3,4,5].A significant proportion of patients with COVID-19 require respiratory support, mechanical ventilation, and long-term use of high doses of sedatives, most of which should be considered in the context of the unique pathophysiology of COVID-19 and associated neurological disorders [4,6,7]. In addition, it is necessary to consider the long-term consequences of critical illness in intensive care patients with COVID-19, who will face problems in rehabilitation from cognitive impairment, which can significantly affect their quality of life.Dexmedetomidine is a sedative drug used in intensive care settings with several properties that may provide additional benefit to critically ill patients with COVID-19 who require sedation. Dexmedetomidine reduces the hemodynamic response to intubation and extubation and attenuates the stress response to being in the intensive care unit for COVID-19 infection through an α2-mediated decrease in sympathetic tone. Therefore, it should be possible to continue dexmedetomidine sedation during the stressful period of extubation without worrying about respiratory depression while maintaining hemodynamic stability.
2. Material and Methods
- The study included 333 adult patients (18 years and older) with severe COVID-19 who received treatment in intensive care units of the Republican Specialized Zangiota-2 Hospital for COVID-19 (Tashkent region, Uzbekistan). Among the patients, the following psycho-emotional disorders were identified: depressive syndrome, anxiety disorders, post-traumatic stress disorders (PTSD), as well as various combinations of them.Upon arrival at the intensive care unit, patients were randomized to receive dexmedetomidine or propofol by continuous intravenous infusion under non-invasive ventilation and analgesia, only if clinically needed, with a bolus of morphine. An initial bolus infusion of dexmedetomidine or propofol was given to achieve steady-state plasma concentrations rapidly. The loading dose of infusion dexmedetomidine was 2.5 μg/kg/h for 10 minutes, followed by a maintenance infusion of 0.2-2.5 μg/kg/h into the peripheral vein. Propofol was given as an infusion of 1-3 mg/kg/h. The degree of sedation was measured and recorded hourly using the Ramsay sedation score (RSS). Patients were maintained at an RSS level of more than two by adjusting the sedative regimen. No other sedative or analgesic agents were used.Patients underwent respiratory therapy using non-invasive mechanical ventilation to achieve an acceptable level of gases in the blood. Weaning from mechanical ventilation was carried out according to clinical indications. The sedative infusion was discontinued in preparation for ventilator-off when the patient was alert after achieving spontaneous breathing with pressure support (Ps) <10 cmH2O, tidal volume >6 ml/kg, and respiratory rate (RR) ≥ 10 and <25 breaths/min, and with arterial oxygen pressure (PaO2) ≥100 mmHg. Art. had an inspired oxygen concentration (FiO2) < 40% and a PEEP < 5 cmH2O with stable hemodynamics.Heart rate (HR), blood pressure (BP) and oxygen saturation (SpO2) were monitored continuously. Arterial blood samples were taken for blood gas analysis (pH, PaO2, PCO2, PaO2/FiO2) immediately upon arrival in the ICU and every 2 hours after that. Cardiovascular and respiratory adverse events were defined as changes in blood pressure ≥30% from baseline, bradycardia <55 bpm, tachyarrhythmias, and respiratory rate <10 or >35 breaths/min after weaning.Data are shown as mean (M±m) values, and comparisons were made using an unpaired t-test. Median and interquartile range are for skewed data, and comparisons were made using the Mann-Whitney U-test. RSS values are shown as median and compared using a two-stage method using pooled scores; the area under the curve was calculated for each patient, and comparisons between groups were made using the Mann-Whitney U-test, and p<0.05 was considered significant. All analysis was carried out using the Statistica software package for Windows.
3. Results
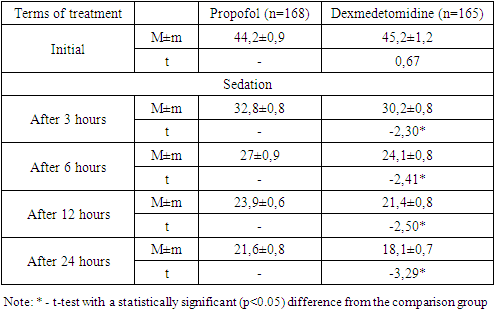
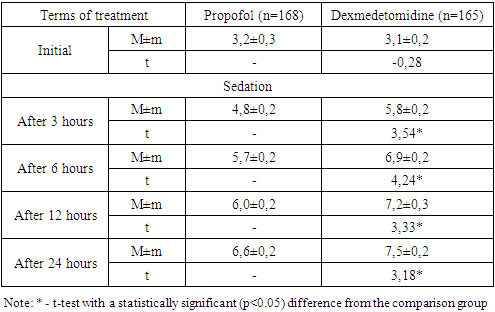
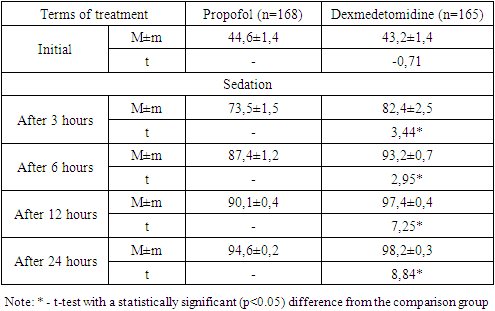
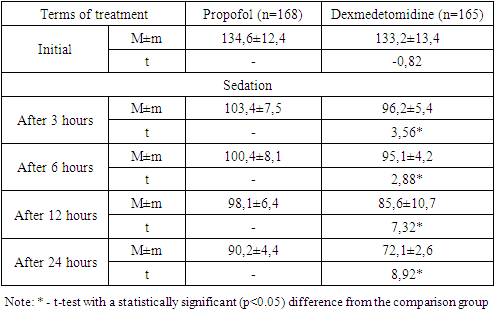
- An analysis of the clinical course of psychoemotional disorders in patients with COVID-19 on the background of intensive care using sedation regimens showed that in the propofol group, in most cases of a combination of depressive syndrome, anxiety disorder and PTSD (12 out of 17; 70.6%), progression of the pathology was noted, while in the main group this indicator was 47.4% (9 out of 19) without a statistical difference (p=0.159).In the dexmedetomidine group, there was a relatively low percentage of cases with deterioration in general conditions. A statistically significant difference in the rates of improvement in the clinic of psychoemotional disorders was noted in cases of the depressive syndrome (p=0.028), anxiety disorders (p=0.018), PTSD (p=0.011), with a combination of depressive-anxiety disorders (p=0.032) and depression / PTSD (p=0.011).As shown in fig. 1 in the dexmedetomidine group, the pooled value of the proportion of progression of the psychologically complicated course of COVID-19 was 21.8% (36 out of 165), which had a statistically significant difference (p<0.001) and was lower than in the propofol group - 47.6% (80 out of 168).In the dexmedetomidine group, the incidence of tracheal intubations was 7.3% (12 of 165), while in the propofol group, this figure was 17.3% (29 of 168). The frequency of intubations was higher in the propofol group and in the early stages of treatment - 1-2 days - 5.36% (dexmedetomidine - 1.8%), 3-4 days - 2.4% versus 1.2%, and later (7-8 days, more than 9 days) periods of stay of patients in the ICU - 5.36% versus 1.8% (Table 2).The terms of treatment in the ICU in patients with depressive syndrome were reduced from 8.5±0.5 to 7.3±0.6 (t=-2.18; p<0.05), in patients with anxiety disorders - from 9.5 ±0.6 to 8.2±0.5 (t=-3.54; p<0.05), in cases of PTSD – from 10.4±0.8 to 8.8±0.5 days (t=-2.86, p<0.05); in extremely severe psychoemotional disorders with a combination of syndromes, a statistically significant difference was also noted in favour of the dexmedetomidine group (Table 3).The average duration of treatment in the ICU in the propofol group was 12.6±0.8 days, while in the dexmedetomidine group, it was 8.4±0.6 (t=-2.89; p<0.05). Patients' length of stay on non-invasive CPAP was also statistically significantly less when sedated with dexmedetomidine, amounting to 5.2±0.4 versus 8.4±0.5 days in the propofol group (t=-5.20; p<0.05).Against the background of sedation and respiratory support in the non-invasive CPAP mode, the dynamics of RR, assessed in a comparative aspect, shows that the initial values were comparable without statistical differences. Subsequently, 3 hours after the administration of the loading dose of the drug and reaching the target depth of sedation, the average RR was 33.7±0.8 per minute in the propofol group and 31.1±0.8 in the dexmedetomidine group (Table 1).
|
|
|
|
4. Conclusions
- In the presence of severe psychoemotional disorders, the effectiveness of protocols in the main areas of etiotropic and pathogenetic treatment of COVID-19 (correction of coagulopathy, respiratory and antibiotic therapy) directly depends on the proper sedation regimen. In this aspect, dexmedetomidine provides adequate and safe respiratory support with improved external respiration, blood gas composition and minimal negative hemodynamic effect.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML