Fakhrutdinova Sevara Srazhitdinovna
Republican Specialized Scientific and Practical Medical Center for Endocrinology named after Academician Y.Kh. Turakulova, Tashkent, the Republic of Uzbekistan
Correspondence to: Fakhrutdinova Sevara Srazhitdinovna, Republican Specialized Scientific and Practical Medical Center for Endocrinology named after Academician Y.Kh. Turakulova, Tashkent, the Republic of Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Premature ovarian insufficiency is considered one of the important health problems of women due to adverse effects on the body, which contribute to a significant increase in the risk of various diseases. Purpose of the Study: The aim of the study is to assess the lipid profile, glucose metabolism and insulin resistance in women with POI with different levels of FSH. Materials and Methods: The main group included 80 women (average age 28.0±7.2 years) with an idiopathic form of POI. The control group included 35 women (average age 30.6±8.3 years) with regular menstruation and no confirmed endocrine disorders. Results: All women with POI were divided into groups according to ESHRE recommendations, 16 women were included in the group with FSH level >25 to 40 mIU/ml, 49 patients were in the group with FSH ≥40 mIU/ml. Fasting plasma glucose, HbAlc, and insulin levels did not differ from those of the control group as a whole and depending on FSH level. The lipid profile in groups with different levels of FSH differed significantly only from the data of the control group. A comparative analysis of the borderline levels of the lipid profile in POI and control groups showed that total cholesterol ≥5.2 mmol/l (χ2=18,9; р <0,0001), TG ≥1,7 mmol/l (χ2=19,5; р <0,0001) and LDL ≥3.5 mmol/l (χ2=9,8; р=0,02) were statistically significantly more common in POI group than in the control group, and in terms of HDL frequency <1.03 mmol/l (χ2=3,5; р=0,32) the groups did not differ significantly from each other.
Keywords:
Premature ovarian insufficiency (POI), Hormonal profile, Metabolic profile
Cite this paper: Fakhrutdinova Sevara Srazhitdinovna, Metabolic Profile of Women with Premature Ovarian Insufficiency, Depending on the Level of Follicle-Stimulating Hormone, American Journal of Medicine and Medical Sciences, Vol. 12 No. 12, 2022, pp. 1297-1302. doi: 10.5923/j.ajmms.20221212.25.
1. Introduction
According to the recommendations of the European Society of Human Reproduction and Embryology (ESHRE), premature ovarian insufficiency (POI) is a clinical syndrome defined as loss of ovarian function in women under 40 years of age, characterized by the presence of oligo-/amenorrhea lasting at least four months with an increase in gonadotropin levels and low content of estradiol. [1].The cause of POI is still a matter of debate among scholars. It is assumed that genetic disorders, hormonal and metabolic changes, infections and autoimmune diseases, as well as iatrogenic causes (surgery, radiation therapy and chemotherapy) can contribute to the disease. [2].It is estimated that the prevalence of POI ranges from 0.01% in women under 20 years of age to 1% of women under 40 years of age and varies depending on the ethnicity and age of women. [2,3,4]. The disease is more common in women of Caucasian race (1-1.4%), African -American and Latina women. [5]. Residents of China (0.5%) and Japan are less susceptible to this pathology (0,1%) [6].Incidence of POI in women with primary amenorrhea is 10–28%, while in women with secondary amenorrhea- it is 4–18% and up to 5% of endocrine infertility. [5,7].Premature ovarian insufficiency is considered one of the important health problems of women due to adverse effects on the body, which contribute to a significant increase in the risk of cardiovascular disease (CVD), osteoporosis and neurological disorders. [8,9].
2. Purpose of the Study
The purpose of the study is to evaluate the lipid profile, glucose metabolism and insulin resistance in women with POI with different FSH levels.
3. Materials and Methods
80 women (average age 28.0±7.2 years) with an idiopathic form of POI were included in the main group. 35 women (average age 30.6±8.3 years) with regular menstruation and no confirmed endocrine disorders were included in the control group. All women in the study signed an informed consent to an anonymous analysis of their medical data.According to ESHRE recommendations, the diagnostic criteria for POI are: oligo / amenorrhea for at least 4 months and increased FSH level >25 IU / l in two cases with an interval of >4 weeks [1].Blood sampling was performed in the follicular phase (3rd–5th day of the menstrual cycle) in controls and against the background of amenorrhea in women with suspected POI.The study of the lipid spectrum of blood was carried out by the photometric method using reagent kits from "Human" company (Germany). The levels of hormones in the blood serum were determined (FSH, LH, estradiol (E2), prolactin, AMH, SHBG, DHEAS, testosterone, TSH, free T4, TPOAb) by the electrochemiluminescent method on the immunochemical analyzer Elecsys and cobas e. s using standard kits Cobas Roche ("Roche Diagnostics GmbH", Germany).Determination of glucose was carried out by the glucose oxidant method using kits from "Human" company (Germany). Glycosylated hemoglobin (HbA1c) was determined by the turbidimetric method with reagents kit from Human (Germany). Insulin level was determined by electrochemiluminescent method on the immunochemical analyzer Elecsys and cobas e. using standard kits Cobas Roche (Germany).Insulin resistance index HOMA IR was calculated by the formula: HOMA-IR = fasting insulin * fasting glucose / 22.5. Insulin resistance was established when HOMA-IR >2.5.The normal reference ranges used in our laboratory were as follows: FSH: 3.5–12.5 mIU/ml; LH: 2.4–12.6 mIU/ml; E2: 68–1269 pmol/l; PRL: 4.79–23.3 ng/ml; AMH: 0.09-9.49 ng/ml; SHBG: 32.4–128.0 nmol/l; DHEAS: 60.9–407.0 mcg/dl; T: 0.29–1.67 nmol/l; TSH: 0.27–4.0 mIU/ml; fT4: 0.93–2.0 ng/dl; TPOAb <34.0 IU / ml.
4. Statistical Analysis
SPSS 23.0 was used for statistical analysis. The single-sample Kolmogorov-Smirnov test was used for normality of distribution. Continuous variables that were not normally distributed were presented as median (quartile interval) and compared by nonparametric test. ROC analysis was conducted using interactive programs easyROC, ver. 1.3 (http://www.biosoft.hacettepe.edu.tr/easyROC). To determine a diagnostically significant FSH cut-off point, ROC analysis was used with making ROC curve and the indication of the area under the curve (AUC). The differences were considered statistically significant when р<0.05.
5. Results and Discussion
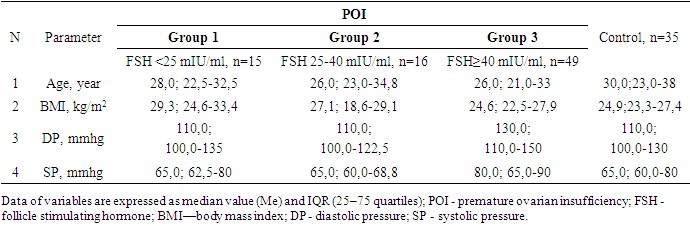
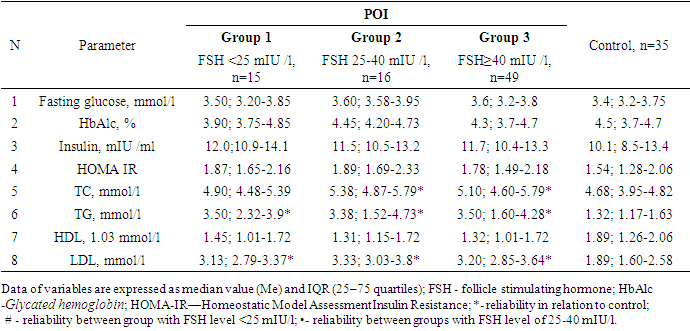
All women with POI were divided into groups based on ESHRE recommendations, FSH group >25 to 40 mIU/ml included 16 women, FSH group ≥40 mIU/ml included 49 patients.Since the maximum reference range of FSH in our laboratory was 12.5 mIU/ ml, the study included women under 40 years of age with menstrual irregularities (secondary amenorrhea lasting 6 months or more), with FSH levels of <25 mIU / ml, high LH levels and low E2 obtained twice with an interval of 4 weeks, AMH concentration was also taken into account. This group of women (n = 15) (average age 28.7±8.0 years) did not take medications (for 6 months before the examination), which could affect hormonal and biochemical indicators.Thus, 3 groups of patients were identified: group 1 - FSH <25 mIU/ml (n=15), group 2- FSH 25-40 mIU/ml (n=16) and group 3-FSH ≥40 mIU/ml (n=49). It should be noted that the majority (61.3%) of women with POI had FSH level ≥40 mIU/ml.It is known that both age and BMI can have an impact on metabolic rates, but our study found no differences in age and BMI between the three groups. The age of the examined patients and persons from the control group ranged from 18 to 45 years old. (Table 1.).Table 1. Characteristics POI and control group
 |
| |
|
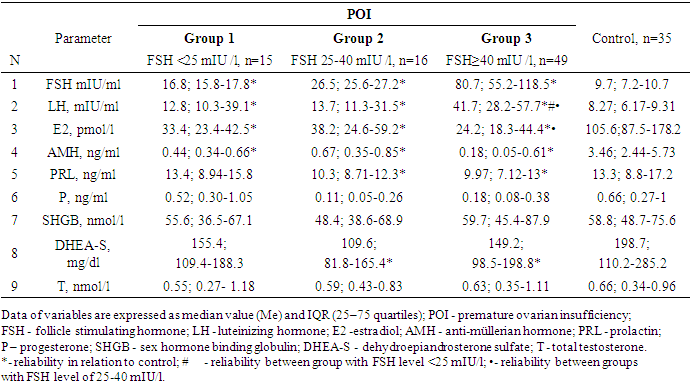
As expected, the levels of FSH, LH were higher in all groups relative to the control, and E2 and AMH were lower. (Table 2.).Table 2. Hormonal and clinical features of patients with POI and the control group
 |
| |
|
LH level is statistically significantly higher in the group with FSH ≥40 mIU / ml compared with patients from FSH groups <25 mIU / ml (p = 0.003) and FSH 25-40 mIU / ml (p<0.0001). Estradiol index was lower in the group with FSH ≥40 mIU/l compared to the group with FSH 25-40 mIU/ml (p=0.03) and unreliably, but also lower than in the group with FSH <25 mIU/mL (р=0,21).As for the other hormones, significant differences were noted only in the groups of FSH 25-40 mIU/l and FSH ≥40 mIU/l in prolactin level (p=0.04 and p=0.003, respectively) and DHEAS (respectively, p=0.01 and р=0,03).Since 15 (18.9%) of 80 women with POI had FSH level lower than recommended by ESHRE (FSH >25 IU/l), and the upper borderline of the reference value of the manufacturer of the hormone test kit was 12.5 mIU/ml, we decided to determine a diagnostically significant cut-off point using ROC analysis with making ROC curve and indication of the area under the curve (AUC). The choice of cut-off value was carried out at the point of the graph with the maximum sum of sensitivity and specificity (Figure 1.). 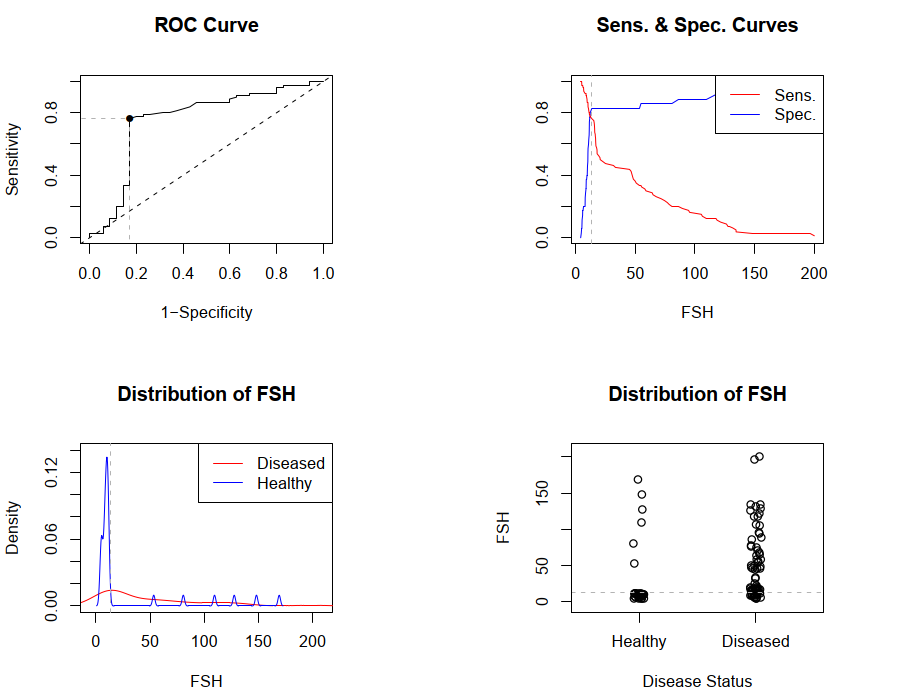 | Figure 1. ROC curves and cut-off point of FSH. FSH - follicle stimulating hormone |
During ROC analysis, it was possible to determine the area under AUC curve = 0.754 (95% CI 0.645-0.864), this indicator proved good quality of the model. The optimal cut-off point for FSH is 13.4 mIU / ml, with Se - 0.762; 95% CI 0.654-0.851 and Sp - 0.914; 95% CI 0.769-0.982. Other parameters were also determined to assess the predictive value of FSH: positive predictive value (0.910; 95% CI 0.806-0.947), negative predictive value (0.604; 95% CI 0.474-0.818), positive likelihood ratio: (4.448; 95% CI 2.12-9.309) and negative likelihood ratio (0.287; 95% CI 0.188-0.436).Thus, FSH cut-off point of 13.4 mIU/ml is the starting point for identifying risk group for POI.Then, we compared the metabolic profile of women with POI depending on FSH level.No differences were found between the groups regarding fasting glucose, HbAlc, insulin and HOMA-IR (Table 3.). Table 3. Metabolic profile of patients with POI and the control group
 |
| |
|
Indicators of the lipid spectrum (TC, TG and LDL) in groups with different levels of FSH differed significantly only from the data of the control group. The lipid profile of women with POI had no differences between groups in terms of FSH levels.If we compare POI group and the control, then total cholesterol ≥5.2 mmol/l (χ2=18,9; р <0,0001), TG ≥1.7 mmol/l (χ2=19,5; р <0,0001) and LDL ≥3.5 mmol/l (χ2=9,8; р=0,02) were statistically significantly more common in POI than in the control group, and by HDL frequency <1.03 mmol/l (χ2=3,5; р=0,32) the groups did not differ significantly from each other (Figure 2).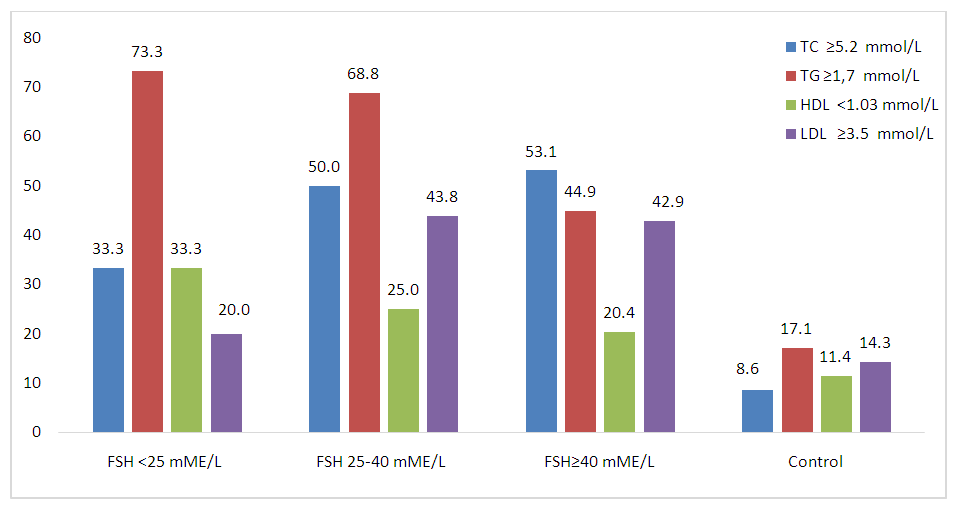 | Figure 2. Frequency of occurrence of lipid spectrum indicators that differ from the reference values. FSH - follicle stimulating hormone; TC—total cholesterol; TG—triglycerides;HDL—high-density lipoprotein cholesterol; LDL—low-density lipoprotein cholesterol |
A number of authors have suggested that loss of ovarian function and subsequent endogenous estrogen deficiency in women with POI may contribute to a higher risk of cardiovascular disease (CVD) and death. [10,11].As a rule, patients with POI have several risk factors for cardiovascular disease: autonomic and endothelial dysfunction, dyslipidemia, and insulin resistance. Consequently, women with this disease are at a higher risk of developing metabolic syndrome. The risk of mortality from coronary artery disease in patients with POI increases by approximately 80% compared with women with menopause aged 49-55 years [10,11].Knauff E. et al. [12] compared the lipid profile of women with and without POI. All women were not taking hormone replacement therapy or oral contraceptives at the time of the study. Also, women in both groups did not differ in BMI, waist circumference, and smoking status. There were no significant differences in total cholesterol or LDL levels. However, in POI group, triglycerides level was significantly higher and HDL cholesterol level was significantly lower than in women without POI. The authors believe that changes in TG levels were probably causally related to the lack of endogenous ovarian function. However, it is not clear why women with POI have higher triglyceride levels and potentially lower HDL levels, which are characteristic of insulin resistance but differ from the lipid changes commonly seen in women with menopause later in life.Harchaoui K. et al. [13] have found that even a slight increase in basal FSH level in women (defined as FSH > 7 IU/l) may be associated with higher serum cholesterol concentrations.Kalantaridou S. et al. [14] studied the metabolic profile of 18 patients with POI, especially fasting serum glucose level, and concluded that there was no significant difference compared to 20 healthy people in the control group. In turn, Ates S.et al. [15] studying glucose metabolism did not reveal significant differences in insulin sensitivity between patients with POI and healthy controls. In addition, it was found that the overall percentage of women with POI had abnormal fasting glucose concentrations (21% vs. 9% in control) but they had lower rates of insulin resistance (25% vs. 37%). Moreover, it was found that HOMA-IR values correlated positively with BMI in healthy people, but not in POI group.On the contrary, Kulaksizoglu M. et al. [16] described an increase in serum glucose and insulin concentrations leading to abnormal HOMA-IR in patients with POI. Corrigan E. et al. [17] also reported decreased insulin sensitivity in patients with POI compared to healthy but more full-figured people in control group.Gulhan I. et al. [18] found high levels of TC and LDL (p=0.006 and p=0.040, respectively) in women with POI. However, no differences were found between groups in terms of TG and HDL levels (p= 0.128 and p = 0.062, respectively). It is noted that in the group with POI there is a significant negative correlation between the levels of E2 and TG (r = -0.291, p = 0.047). The authors believe that higher levels of TC and LDL in women with POI compared to the control group suggest that estrogen deprivation in women with POI leads to adverse changes in lipid profile.According to Kunicki M. et al. [3], lowering FSH threshold to 25 IU/l (according to the latest definition of POI) does not affect glucose or lipid metabolism, but does affect serum LDL concentration. This rate was highest in women with FSH level >40 IU/l.The pathological mechanism of this phenomenon is explained by Song Y. et al. [19], who suggest that signaling through intrahepatic FSH receptors can reduce the number of LDL receptors, contributing to the impaired LDL uptake by hepatocytes, which consequently leads to an increase in serum LDL level.Thus, data on the metabolic profile of women with FDI are contradictory.
6. Conclusions
1. Diagnostically significant cut-off point for FSH was determined with the help of ROC analysis. FSH cut-off point of 13.4 mIU/ml is the starting point for identifying POI risk group.2. Fasting glucose, HbAlc and insulin levels did not differ from indicators of the control group as a whole and depending on FSH level. The lipid profile in groups with different levels of FSH differed significantly only from the data of the control group. Lipid spectrum indicators (TC, TG and LDL) in women with POI did not differ depending on FSH level. 3. Comparative analysis of the borderline levels of the lipid profile in POI and control groups showed that total cholesterol ≥5.2 mmol/l (χ2=18.9; p<0.0001), TG ≥1.7 mmol/l (χ2= 19.5; p<0.0001) and LDL ≥3.5 mmol/l (χ2=9.8; p=0.02) were statistically significantly more common in POI than in the control group, and by frequency of HDL <1.03 mmol/l (χ2=3.5; p=0.32) the groups did not differ significantly from each other.Conflict of interest: The author has no conflict of interest to declare.
References
| [1] | Webber L., Davies M., Anderson R. et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31: 926–937. doi: 10.1093/humrep/dew027. |
| [2] | Podfigurna A., Stellmach A., Szeliga A. et al. Metabolic Profile of Patients with Premature Ovarian Insufficiency. J Clin Med. 2018; 7(10): 374. doi: 10.3390/jcm7100374. |
| [3] | Kunicki M., Kruszewska J., Skórska J. et al. Does the Value of FSH Predict Severity of Metabolic Complications in Females with POI? J Clin Med. 2022; 11(7): 2024. doi: 10.3390/jcm11072024. |
| [4] | Lagergren K., Hammar M., Nedstrand E. et al. The prevalence of primary ovarian insufficiency in Sweden; a national register study. BMC Women's Health. 2018; 18: 75. doi: 10.1186/s12905-018-0665-2. |
| [5] | Luborsky J., Meyer P., Sowers M. et al. Premature menopause in a multi-ethnic population study of the menopause transition. Hum. Reprod. 2003; 18: 199–206. doi: 10.1093/humrep/deg005. |
| [6] | Kalu E., Panay N. Spontaneous premature ovarian failure: management challenges/ Kalu Gynecol Endocrinol. 2008; 24(5): 273-279. doi: 10.1080/09513590801990764. |
| [7] | O’Flynn N. Assessment and treatment for people with fertility problems: NICE guideline. Br. J. Gen. Pract. 2014; 64(618): 50-51. doi: 10.3399/bjgp14X676609. |
| [8] | Louwers Y., Visser J. Shared Genetics Between Age at Menopause, Early Menopause, POI and Other Traits. Front Genet. 2021; 12: 676546. doi: 10.3389/fgene.2021.676546. |
| [9] | Stevenson J., Collins P., Hamoda H. et al. Cardiometabolic health in premature ovarian insufficiency. Climacteric. 2021; 24(5): 474-480. doi: 10.1080/13697137.2021.1910232. |
| [10] | Podfigurna-Stopa A., Czyzyk A., Grymowicz M. et al. Premature ovarian insufficiency: the context of long-term effects. J Endocrinol Invest. 2016; 39(9): 983-990. doi: 10.1007/s40618-016-0467-z. |
| [11] | Wellons M. Cardiovascular disease and primary ovarian insufficiency. Semin Reprod Med. 2011; 29(4): 328-341. doi: 10.1055/s-0031-1280918. |
| [12] | Knauff E., Westerveld H., Goverde A. et al. Lipid profile of women with premature ovarian failure. Menopause. 2008; 15(5): 919-923. doi: 10.1097/gme.0b013e31816b4509. |
| [13] | Harchaoui K., Visser M., Kastelein J. et al. Triglycerides and Cardiovascular Risk. Curr. Cardiol. Rev. 2009; 5: 216–222. doi: 10.2174/157340309788970315. |
| [14] | Kalantaridou S., Naka K., Papanikolaou E. et al. Impaired endothelial function in young women with premature ovarian failure: Normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004; 89: 3907–3913. doi: 10.1210/jc.2004-0015. |
| [15] | Ates S., Yesil G., Sevket O. et al. Comparison of metabolic profile and abdominal fat distribution between karyotypically normal women with premature ovarian insufficiency and age matched controls. Maturitas. 2014; 79: 306–310. doi: 10.1016/j.maturitas.2014.07.008. |
| [16] | Kulaksizoglu M., Ipekci S., Kebapcilar L. et al. Risk factors for diabetes mellitus in women with primary ovarian insufficiency. Biol Trace Elem. Res. 2013; 154: 313–320. doi: 10.1007/s12011-013-9738-0. |
| [17] | Corrigan E., Nelson L., Bakalov V. et al. Effects of ovarian failure and X-chromosome deletion on body composition and insulin sensitivity in young women. Menopause. 2006; 13: 911–916. doi: 10.1097/01.gme.0000248702.25259.00. |
| [18] | Gulhan I., Bozkaya G., Uyar I. et al. Serum lipid levels in women with premature ovarian failure. Menopause. 2012; 19: 1231–1234. doi: 10.1097/gme.0b013e318254102b. |
| [19] | Song Y., Wang E.-S., Xing L.-L. et al. Follicle-stimulating hormone induces postmenopausal dyslipidemia through inhibiting hepatic cholesterol metabolism. J. Clin. Endocrinol. Metab. 2016; 101: 254–263. doi: 10.1210/jc.2015-2724. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


