Mamatova Muborak Nurpulatovna1, Alamova Feruza Sayfiddinovna2
1Doctor of Biological Sciences, Action Professor, Samarkand State Medical University, Samarkand, Uzbekistan
2Assistant, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Mamatova Muborak Nurpulatovna, Doctor of Biological Sciences, Action Professor, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Rabies is a particularly dangerous infectious disease of animals, birds and humans, which occurs with severe damage to the nervous system, usually fatal. Rabies is known in Asia since ancient times, and epizootics of this disease among domestic animals was described in India, Iran and China since two thousand years BC. But despite the successes of modern science, the vast territory of the globe is not free from the rabies virus1. The global range of rabies in productive animals does not yet have a tendency to decrease due to the natural foci of the disease2. The problem of eliminating rabies is considered the most urgent task of local and foreign infectious disease specialists [2,3,7,8,9,10]. Stray dogs are of great importance in the spread of rabies among people, while wild carnivores play the main role among farm and domestic animals [11,12,13,14].
Keywords:
Infectio, Rabies, Lissavirus, Antirabic vaccine, Innovation texnologiy
Cite this paper: Mamatova Muborak Nurpulatovna, Alamova Feruza Sayfiddinovna, Biological Properties of Rabies Virus and Immunogenicity of Oral Antirabic Vaccines in Granules, American Journal of Medicine and Medical Sciences, Vol. 12 No. 12, 2022, pp. 1195-1200. doi: 10.5923/j.ajmms.20221212.05.
1. Introduction
In recent years, we have regularly observed cases of rabies among cattle and dogs, as well as among foxes. Natural foci of rabies have become more active in our Republic. To clarify the nature of natural epizootics and to successfully solve the problem of rabies disease, as well as to develop preventive measures and provide the control of animal rabies, not only the study of host ecology, but also data on the study of the biological characteristics of the rabies virus isolated in a particular geographical area is important. In view of the crucial importance of the antigenic and pathogenic properties of the virus, we set ourselves the task of studying isolates allotted from different geographical areas of Uzbekistan.
2. Analysis and Results
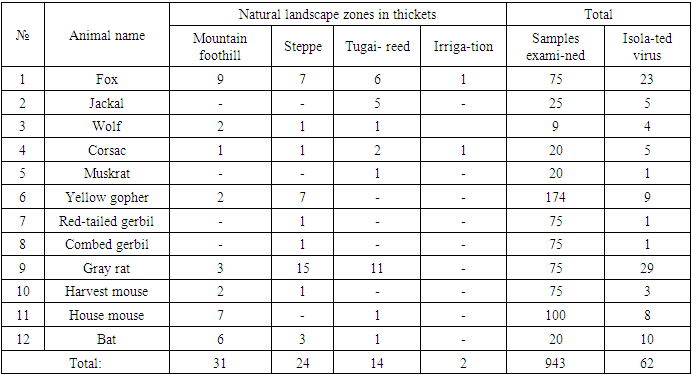
In 2008-2018, 1,787 samples of material from animals suspected of rabies (brain, cerebellum, salivary gland samples) were analysed using comprehensive diagnostics, i.e. luminescent microscopy and bioassay on mice. The rabies virus was detected in 157 (8.8%) samples. 10 isolates of street rabies virus from different animal species inhabiting different natural and climatic zones of Uzbekistan was selected for further study from its total number.Table 1. Results of rabies virus isolation from wild carnivorous animals and rodents settled in different natural zones of Uzbekistan for 2008-2018
 |
| |
|
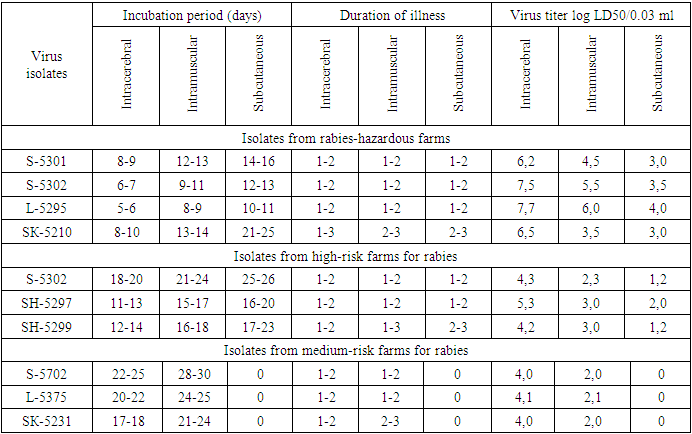
From the data presented in the table, it can be seen that in different natural zones of Uzbekistan, the rabies virus circulates among wild animals - often in foxes and gray rats.In recent years, it has been established that wild animals are the main carriers of the virus, thereby creating natural foci of rabies, i.e. especially hazardous areas for rabies. The isolation of the rabies virus from rodents, in particular, from gray rats living in livestock buildings and yards, indicates that they are the main potential source of this infection.According to morphological, antigenic and other indicators, the identified virus isolates are assigned to the family of rhabdoviruses, the genus of lyssaviruses and the species - rabies virus.Determination of study virus titre on white mice and its calculation according to Reed and Mench method revealed high titres of 6.2-7.7 log LD50/0.03 ml in isolates obtained from foxes, grey rats, delivered from farms of particularly dangerous (stationary rabies-unsafe) zones.In experiments, we studied the sensitivity of white mice weighing 10-12 g to infection with a street virus. A total of 10 street virus isolates were selected for study.We used a 10% suspension of infected mouse brain prepared in sterile saline. In experimental mice, the suspension was injected at a dose of 0.03 ml intramuscularly and 0.1 ml subcutaneously.Strain LM-5301 was isolated from the brain of a stray dog (three passages in mice); strain S-5302 was isolated from the brain of a clinically healthy dog (four passages in mice); strain L-5271 was isolated from the brain of a fox (two passages in mice); strain L-5295 was isolated from the brain of a fox (two passages in mice); strain L-5375 was isolated from the brain of a fox (three passages in mice); strain W-5297 was isolated from jackal brain (three passages in mice); strain W-5299 was isolated from the brain of a jackal (two passages in mice); strain SK-5210 was isolated from the brain of gray rats (three passages in mice); strain SK-5231 was isolated from the brain of a gray rat (three passages in mice).Studies on the detection of the sensitivity of white mice to infection with street rabies virus by various methods are presented in Table 2.Table 2. Susceptibility of white mice to infection with street rabies virus by different methods
 |
| |
|
The data in the table show that in the case of rabies virus infection, the duration of the incubation period of the disease in mice and the infectious titer of the virus depend on the method of inoculation.In this experiment, white mice were relatively highly susceptible when they were infected intracerebally.The results of these studies on mice allowed the isolates to be divided mainly into two groups:a) Isolates isolated from rabies-hazardous farms with short incubation periods (5-10 days) and high infectious titres (6.2-7.7 log LD50/0.03 ml);b) isolates isolated in high-risk farms for rabies, with long incubation periods (11-20 days) and low infectious titer (4.0-4.1 log LD50/0.03 ml);c) isolates isolated in medium-risk farms for rabies, with long incubation periods (17-25 days) and low infectious titer (4.0-4.1 log LD50/0.03 ml).Based on the conducted studies, isolates extracted from carnivores and rodents in natural rabies hotspots, i.e. isolated from rabies-hazardous farms, are relatively highly pathogenic for guinea pigs and polar mice under different modes of infection.Of the 10 field isolates of rabies virus isolated from dogs, foxes and grey rats, 4 isolates tended to be more pathogenical for white mice and 3 isolates for guinea pigs.Thus, a comparative study of the pathogenic activity of the street rabies virus for guinea pigs and white mice isolated from wild animals made it possible to divide the studied isolates into three groups of rabies virus strains:1) highly active isolates isolated on the territory of rabies-hazardous farms;2) active isolates isolated on the territory of high-risk farms for rabies;3) weakly active isolates isolated on the territory of medium-risk farms for rabies.The conducted scientific work demonstrated that foxes are currently the main natural focal sources of infection, while gray rats are carriers of the rabies virus. The intensity and seasonality of the epizootic process among farm and domestic animals is primarily associated with the ecology of wild carnivores.A number of issues related to this disease have not yet been clarified, and among them the improvement of preventive measures due to the spread of rabies among wild carnivores is especially important.
3. The Actuality of the Problem
All types of mammals, poultry and human beings are down with hydrophobia disease. The disease is caused by filtered neurotopic virus. The virus badly damages the cerebrum of animal [1,2].Basically, this disease infects through saliva when an animal is bite. In this case one should be pointed out, that the virus in reserved period, that is before 10 days of revealing clinic symptoms of the disease, exits in tears and saliva of infected animal. Therefore, if human beings or healthy animals are bite by rabid dog, cat, fox, wolf or rodents, in spite of the fact that the symptoms of hydrophobia are revealed, they should be vaccinated against hydrophobia [3,5,6]. Stability of the virus in nature is considered wild animals and it spreads via dogs and cats. In Uzbekistan environment the most dangerous animals for agriculture and domestic animals are considered vagrant dog, cat and foxes. Some problems of this disease, especially, prevention of spreading hydrophobia among wild animals is still not solved. Many scientists recommended to use several eatable granular antirabic vaccines through mouth in order to prevent the hydrophobia disease among wild animals [4,5].As the result of long year researches and investigations in virusology laboratory of Scientific research institute of Veterinary is achieved to create the granular antirabic vaccination through mouth for wild animals, dogs and cats. The vaccine is determined as not harmful, not reactogen, and such issues as the most appropriate dosage of the vaccine, availability and after one vaccination immunity duration in animals is 6 months are defined. Naturally, this period is not satisfactory for the veterinarian specialists. Therefore, on the base of increasing the main source of vaccine the fix-virus titre improves epizootologic efficiency of vaccine and the process of preparation is set as a goal.
4. Materials and Methods
In order to vaccinate wild animals, dogs, and cats through mouth in laboratory conditions the improved granular antirabic vaccination is prepared in the form of pills. The vaccine consists of meat, flour, apple pectin, gelatin, and the fabric of brain of rapid donkey. The quantity of elements which contains it is “now-how”. In order to discover the immunogenicity of this vaccine 21 1-year dogs divided into 6 groups: 12 dogs (4 dogs per 3 groups – experiment) and 9 dogs (3 dogs per 3 groups – control) are experimented in the laboratory of institute. The 12 dogs in experiment groups are given the improved granular antirabic vaccine. The dogs ate the vaccines with keen appetite. The 9 dogs (3 dogs per 3 groups) were served as control groups. They were not given the vaccine. The 12 dogs in experiment groups were given per one pill of granular antirabic vaccine. The blood serum was taken from the dogs which were both in experiment and control groups before vaccination and after 3, 6, 9, and 12 month of vaccination and checked for appeared virus of neutralizing antibody titre against to virus of hydrophobia in neutralizing reaction. The blood serum which was taken from dogs were liquefied in proportions of 1:2, 1:4, 1:8, 1:12, 1:16, 1:24, 1:32, 1:48, 1:64, and 1:128 to set neutralizing reaction. 12 dogs in experiment after 6, 9, and 12-month vaccination and 3 dogs in control group of this period, in total titres of 9 dogs are 10-4,58 log. LD50 0,03 ml. were infected into chewing muscle with “field virus” at the same periods in amounts of 12 ml. and according to the results the effectiveness of vaccine was estimated. All dogs in control (vaccinated) after infection of hydrophobia were burnt in crematoria of laboratory.
5. The Results of Experiment
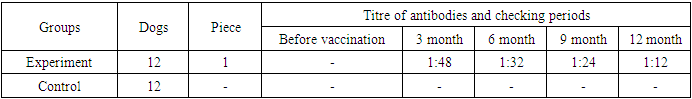
The blood serum of the dogs in experiment group before vaccination when was checked for antibody titre against to hydrophobia in neutralizing reaction, the virus neutralizing antibody against to the virus of hydrophobia non-registered in their blood serum at all. The blood serum of the dogs which ate per one pill of antirabic vaccine in experiment group when were checked in neutralizing reaction after 3 months of vaccination, the titre of antibody against to the virus of hydrophobia are showed 1:48, after 6 months 1:32, after 9 months 1:24, and after 12 months 1:12. (Table 3). In the blood serum of non-vaccinated dogs in control groups the antibodies against to virus of hydrophobia at the same periods non-registered.Table 3. The results of the neutralizing reaction for checking immunogenicity of the improved granular antirabic vaccine in the form of pill at the dogs
 |
| |
|
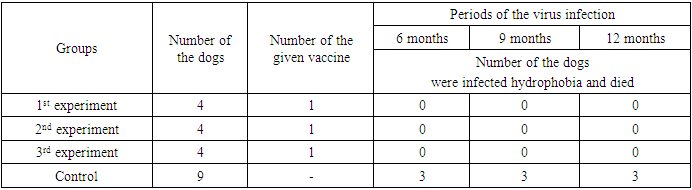
In order to determine the immunity duration of the improved granular antirabic vaccine which was vaccinated through mouth of 4 dogs in the 1st experiment group and after 6 months were infected hydrophobia with titre 104,63 LD50 0,03ml of “field” virus in the amount of 2 ml. within meat. The dogs in the experiment group were observed every day. In the 24th day in 1 dog and in the 25th day in 2 dogs in control groups after infection, were observed clinic symptoms of hydrophobia and soon they are died. But dogs which were vaccinated with “field” virus weren’t infected hydrophobia even after 2 months. (Table 4.) after 9 months of the experiment, 4 dogs in the 2nd experiment group which were vaccinated once with the granular antirabic vaccine and 3 dogs in the control group which were not vaccinated, in order to determine the immunity duration of the vaccine were infected with the hydrophobia “field” virus with titre 104.63 LD50 0,03 ml in the amount of 2 ml within meat. After 17 days 1 dog of infection and in the 20th day 2 dogs in the control group (which were not vaccinated) had hydrophobia and were died.Table 4. The results of the experiment of the immunogenicity of the improved granular antirabic vaccine in the form of pill at the dogs after 6 months
 |
| |
|
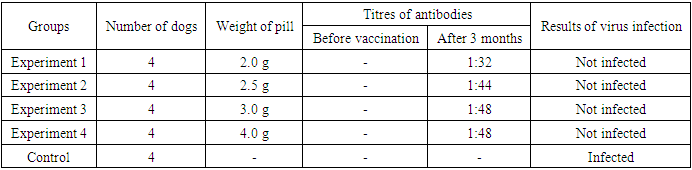
But after 62 days of infection with “field” virus vaccinated dogs were not infected hydrophobia. After 12 months of the experiment 4 dogs which were vaccinated through mouth in 3rd experimental group and 3 dogs which were not vaccinated in control group were infected with hydrophobia “field” virus in the amount of 12 ml within meat which was mentioned above. After 17 days of infection 1 dog and in the 18th and 19th days per 1 dog, in total in 3 dogs of control group which were not vaccinated, were observed clinic symptoms of hydrophobia and soon they were died, but vaccinated dogs after 2 months of infection with “field” virus were not infected hydrophobia. It was determined that, in the case of giving once to dogs of the improved granular antirabic vaccine which was created in laboratory conditions has 12 month of high immunogenicity.In order to determine the best appropriate dosage of the improved granular antirabic vaccine created in laboratory conditions were held an experiment on 20 dogs. The dogs divided into 5 groups, 16 of them served as experimental groups and 4 dogs served as control group. 4 dogs of the 1st experimental group was given per 2,0 grams one of granular antirabic vaccine, 4 dogs in 2nd group are given per 2,5 grams one, 4 dogs of 3rd experimental group were given per 3,0 grams one and to the dogs of the 4th group were given per 4,0 grams one of granular antirabic vaccine. The blood serum was taken from the dogs of both experimental and control groups before and after 3 months of vaccination, and the degree of antibodies were determined in neutralizing reaction. (Table 5).Table 5. The results of checking the best appropriate dosage of granular antirabic vaccine on dogs
 |
| |
|
When the blood serum of the dogs of all 4 experimental groups after 3 months of vaccination was checked in neutralizing reaction and contained the titre of antibodies against to hydrophobia virus in the 1st group – average 1:32, in 2nd group – average 1:44, in 3rd group – average 1:48, and in the 4th group – average 1:48. In the blood serum of not vaccinated dogs of control group the antibodies against to the hydrophobia virus are not registered at that periods.In order to determine the best appropriate dosage of the granular antirabic vaccine which is vaccinated through mouth, after vaccination 20 dogs of both experimental and control groups were infected with hydrophobia “field” virus with titre 104.63 LD50 0,03 ml in the amount of 2 ml within meat. After 19 days of infection 2 dogs and in the 21st day 2 dogs from control group (not vaccinated) had clinic symptoms of hydrophobia and were died, but vaccinated all 4 groups of dogs after 65 days of infection were not infected with hydrophobia. In spite of all vaccinated experimental groups of dogs after 3 months of infection were not infected with hydrophobia, the best appropriate dosage of vaccination is determined 3,0 grams of vaccine, because of the titre of neutralizing antibodies were low in dogs which were vaccinated with 2,0 grams of vaccine.In order to determine the active saving period of the granular antirabic vaccine, after 6 months of preparation of the vaccine were held an experiment on 8 dogs. 4 dogs (1st group) in experiment were given one (ternary) antirabic vaccine, 4 dogs in control group were not given vaccine. Non-vaccinated dogs in control group and vaccinated dogs in experimental group after 6 months were infected with hydrophobia “field” virus with titre 10-4,63 LD50 0,03 ml within meat, and are experimented the degree of immunity of dogs.After 18-20 days of virus infection in 2 dogs from control group (non-vaccinated) were observed clinic symptoms of hydrophobia and they were died, but vaccinated 4 dogs after 65 days of infection were not infected with hydrophobia. So, the active saving period of the granular antirabic vaccine is proved 6 months.Table 6. The results of experimenting active saving period of the granular antirabic vaccine on dogs
 |
| |
|
6. Conclusions
The immunogenicity of the improved granular antirabic vaccine in the form of pill after vaccination once through mouth lasts 12 months is proved in held experiments. The best appropriate dosage of this vaccine is 3,0 grams and the active saving period of the vaccine is 6 months are scientifically proved by experiments.To solve this problem, for the first time, a modified technology for the manufacture and control of an anti-rabies vaccine in the form of a dragee for oral immunization of wild carnivores and rodents was developed, besides this technology is characterized by high economic efficiency, as well as ecological advantages for wild fauna.Thus, the new oral anti-rabies vaccine in the form of pellets made of local raw material by new technology is ecologically safe and harmless, more economical and has 100% efficiency than previously recommended vaccines in pellets, and meets the requirements of international standard for medicines.
Notes
1. През World 1Organization for Animal Health (2020) - World Animal Health Information Database (WAHID).2. Available at: www.oie.int/wahis; http://www.oie.int/en/animal-health-in-the-world/rabies-portal/.
References
| [1] | Борисов А.В., Гусева М.Н. Хранение вируса бешенства с различными стабилизаторами // Мат. межд. научно-практ. конф. ВНИИВВиМ. 30-31 мая 2001. -С. 61-63. |
| [2] | Вагабов Р.М, Баринский И.Ф. Вирусы группы бешенства // Вопросы вирусологии. -М., 1979. -№ 5. -С. 451-458. |
| [3] | Кантарович Р.А. Вирусы группы бешенства // Микробиология, эпидемиология и вирусология. -М.: Медицина, 1972. -№ II. -С. 9-15. |
| [4] | Маматов Н.М. Сравнительное изучение скорости проникновения вируса бешенства в ЦНС и значение места инфицирования в развитии заболевания. Болезни сельскохозяйственных животных. Тр.Уз.НИВИ, 1978, Т.27, -С.71-74. |
| [5] | Маматова М.Н. Эпизоотология бешенства и способы пероральной иммунизации плотоядных животных // Автореферат диссертации на соискание ученой степени кандидата вет. наук. –Ташкент, 1996. -С. 77-97. |
| [6] | Метлин А.Е., Рыбаков С.С. Выделение антигена вируса бешенства в консервированных формалином пробах головного мозга мышей // Мат. межд. научно-практ. конф. ВНИИВВиМ. 30-31 мая 2001. -С. 46-49. |
| [7] | Официальный сайт Всемирной Организации Здравоохранения. 2018. [Эл.ресурс]. Режим доступа: http://www. who.int/mediacentre /factsheets/fs099/ru/ |
| [8] | Аvailable at: www.oie.int/wahis; http://www.oie.int/en/animal-health-in-the-world/rabies-portal/. |
| [9] | International Committe on Taxonomy of Viruses [electronic resource]. - URL: http://www.ictvonline.org/virusTaxonomy.asp. - 13.11.2013. -Р. 177. |
| [10] | Rabies - World Health Organization (WHO). 2020. https://www.who.int/health-topics/rabies#tab=tab_1. |
| [11] | Terrestrial Animal Health Code Chapter 7.7, 6th Edition. World Organisation for Animal Health (OIE), France, 2017. Available at http://www.oie.int/en/international-standard-setting/terrestrial-code/access-online/, accessed March 2018. |
| [12] | WHO. 0 by 30 our catalytic response. Available at http://www.who.int/rabies/United_against_Rabies/en/, accessed December 2017. |
| [13] | WHO Expert Consultation on Rabies, third report: WHO Technical Series Report No. 1012, Geneva, 2018 (ISBN 978-92-4-121021-8). |
| [14] | WHO fact sheet on rabies. Available at http://www.who.int/mediacentre/factsheets/fs099/en/, accessed March 2018. 5 Birhane MG. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




