-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(11): 1171-1174
doi:10.5923/j.ajmms.20221211.16
Received: Nov. 12, 2022; Accepted: Nov. 26, 2022; Published: Nov. 29, 2022

Biochemical Aspects of Fetal Amniotic Fluid Against the Background of Maternal Coronavirus Infection
U. U. Jabborov, Y. K. Nasirov
Republican Perinatal Center, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the Republican Perinatal Center in 2022, a wide specific and non-specific biochemical analysis of amniotic fluid was carried out in 30 pregnant women who were delivered during this time. Of these, all pregnant women are divided into 2 groups. Group I (control) – pregnant women with physiological pregnancy and without a burdened obstetric and somatic history (n=10), group II (main) - pregnant women who underwent COVID–19 during their 2nd and 3rd trimester gestation (n=20). Amniotic fluid sampling was performed using transabdominal amniocentesis in the period from 22 to 38 weeks of gestation. In the group of pregnant women who underwent COVID-19, there is a decrease in the level of total protein, alpha-amylase, alkaline phosphatase and cholesterol, and from specific indicators there is a decrease in the content of phospholipids and lamellar bodies in the amniotic fluid.
Keywords: Amniotic fluid, Biochemical markers, COVID-19
Cite this paper: U. U. Jabborov, Y. K. Nasirov, Biochemical Aspects of Fetal Amniotic Fluid Against the Background of Maternal Coronavirus Infection, American Journal of Medicine and Medical Sciences, Vol. 12 No. 11, 2022, pp. 1171-1174. doi: 10.5923/j.ajmms.20221211.16.
Article Outline
1. Introduction
- Since the WHO declared a global pandemic of SARS-CoV-2, the virus responsible for COVID-19 has spread to 223 countries with over 588 million cases as of August 2022. The US has the highest number of SARS-CoV-2 infections and deaths associated with COVID-19 and was, in fact, the third leading cause of death in the US in 2020 after heart disease and cancer, with approximately 375,000 deaths reported [1]. SARS-CoV-2 enters host cells by binding the SARS-CoV-2 spike or S(S1) protein to ACE2 receptors in abundance on respiratory epithelium such as type II alveolar epithelial cells [2].According to a systematic review, 6 to 8 percent of pregnant women universally screened for COVID-19 tested positive, 54 to 77 percent of these people were asymptomatic, and pregnant women were more likely to be asymptomatic than non-pregnant people of reproductive age with COVID-19 [3]. Overall, maternal respiratory viral infections have been associated with a higher incidence of several adverse fetal and neonatal outcomes, including IUGR, preterm birth, in some serious cases, even intrauterine fetal death, and neonatal death [4].So far, most large cohort studies have undeniably supported the strong association between COVID-19 during pregnancy and iatrogenic preterm birth [5,6]. Interestingly, an increase in preterm births may be associated with a higher caesarean section rate, which may be the optimal regimen to ensure both maternal and fetal safety in the SARS-CoV-2 infection scenario [7,8].Amniotic fluid is a unique biological environment that reflects the functioning of the fetoplacental complex. At the end of gestation, maternal plasma, fetal membranes, placenta, alveolar contents and fetal urine take part in its formation [9]. Surfactant is synthesized and secreted by type II alveolar epithelial cells, also called pneumocytes, which differentiate between 24 and 34 weeks of gestation in humans. It consists of 70-80% phospholipids, approximately 10% protein and 10% neutral lipids, mainly cholesterol [10].The aim of our study was to study the biochemical markers of amniotic fluid in women who underwent COVID-19 in comparison with the control group to identify indicators characteristic of fetal distress during childbirth.
2. Materials and Research Methods
- 30 pregnant women aged 21 to 40 years of 2-3 trimesters of pregnancy, who were hospitalized at the Republican Perinatal Center in the department of pathology of pregnant women, were examined. All pregnant women during gestation underwent an assessment of non-specific biochemical composition and a specific assessment of surfactant in the amniotic fluid.The patients were divided into 2 groups: group I (control) - pregnant women with physiological pregnancy and without aggravated obstetric and somatic history (n=10), group II (main) - pregnant women who had COVID-19 during their gestation (n =20). The sampling of amniotic fluid was carried out using transabdominal amniocentesis in the period from 22 to 38 weeks of gestation.To determine nonspecific biochemical markers, colorimetric and kinetic research methods were used. Amniotic fluid was pre-centrifuged at 2700 rpm for 5 minutes to separate from cervical mucus, cheese-like lubricant, meconium, epidermal scales and vellus hair of the fetus.The study of amniotic fluid was carried out using a biochemical analyzer RT-1904C (Rayto, China). A wide range of biochemical non-specific parameters were studied, such as: total protein (TP), glucose (GLU), urea (UREA), creatinine (CRE), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), alpha -amylase (AMYL), uric acid (UA), cholesterol (CHOL), triglycerides (TG), low density lipoproteins (LDL), high density lipoproteins (HDL). Biochemical tests were determined using test kits from Cypress Diagnostica, Belgium.To identify specific markers such as phosphatidylcholine (PC), phosphatidylinositol (PI), lysolecithin (LPC) and sphingomyelin (SM) used high performance liquid chromatography (HPLC). which was carried out on an Agilent 1100 series liquid chromatograph (Agilent Technologies Inc., USA) equipped with a G1311A 4-gradient pump, a G1322A degasser, a gradient mixer, a G1314A variable wavelength detector (VWD), and a Rheodyne 7725i loop injector (Rheodyne, USA) with a 100 µl loop. Supelco Discovery HS C18 column (4.6x75 mm/3 µm).The lamellar body of the amniotic fluid was counted using a hematology analyzer. Amniotic fluid before the start of the study was centrifuged at 2700 rpm for 5 minutes to separate from cervical mucus, cheese-like lubricant, meconium, epidermal scales and vellus hair of the fetus.Statistical analysis of the results was performed by conventional methods with the determination of the mean value (M) and the mean error of the arithmetic mean (m) using the Microsoft Excel computer program, with the calculation of Student's t-test to compare the means. Differences were considered statistically significant at a significance level of p<0.05.
3. Results and Discussion
- In the second main group, out of all 20 pregnant women who had coronavirus infection, by parity, 13 (65.0%) were multiparous, and the remaining 7 (35.0%) were primiparous. By gestational age, the majority of women 12 (60.0%) were in the third trimester of pregnancy, and the remaining 8 pregnant women (40.0%) were in the second trimester. According to the anamnesis of pregnant women, as well as the data from the discharge from the hospital where there was hospitalization for COVID-19, in 14 (70.0%) patients the disease proceeded in a moderate form, and in 6 (30.0%) patients in the form of pneumonia, that is, in severe form.As can be seen from Table-1, in the main group of patients who underwent COVID-19, compared with the control group, there was a lower level of total protein, alpha-amylase, alkaline phosphatase and cholesterol. These indicators are the most important for the normal functioning of the feto-placental system, and as a result, the normal growth and development of the fetus.
|
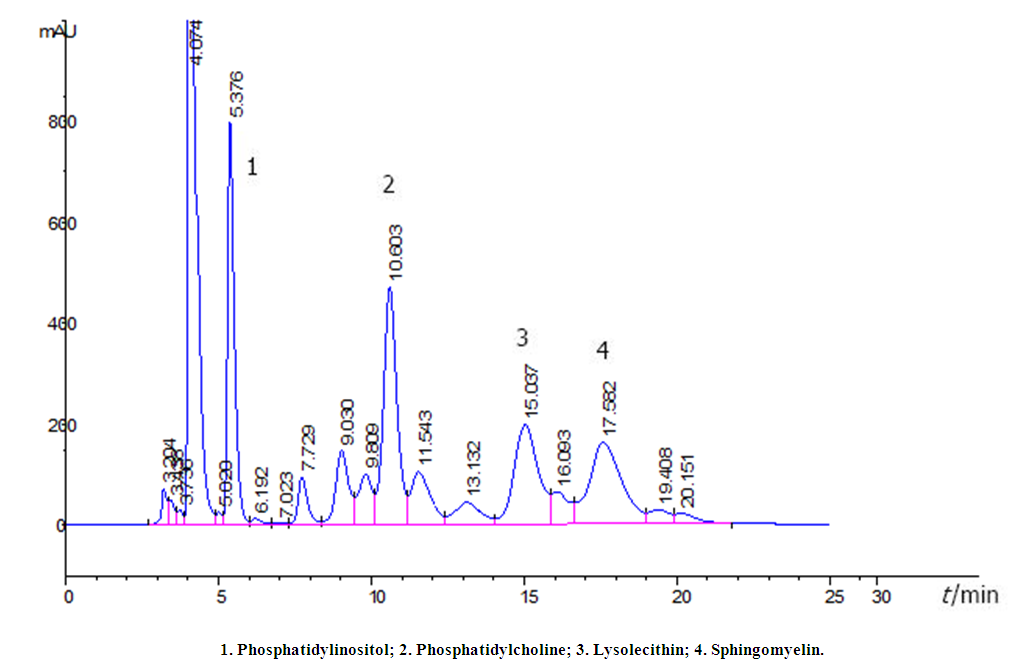 | Figure 1. HPLC of phospholipids extracted from amniotic fluid on a Supelco Discovery HS C18 column (4.6 x 75 mm/3 µm) |
|
4. Conclusions
- 1. The results of non-specific biochemical parameters of the composition of amniotic fluid in patients who underwent COVID-19 indicate a decrease in both total protein, alpha-amylase, alkaline phosphatase, and cholesterol, which suggests that coronavirus infection affects the body of a pregnant woman, the course pregnancy and indicates the occurrence of intrauterine fetal hypoxia.2. The obtained results of specific biochemical parameters of the composition of the amniotic fluid show changes in the content of both phospholipids and lamellar bodies in pregnant women who have undergone COVID-19. A significant decrease in these indicators compared to the control group suggests that the transferred coronavirus infection affects the fetal lung formation system and their maturity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
