-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(11): 1166-1170
doi:10.5923/j.ajmms.20221211.15
Received: Nov. 10, 2022; Accepted: Nov. 26, 2022; Published: Nov. 29, 2022

The Role and Significance of Complement C3 Factor in the Clinical Course of Diabetic Polyneuropathies
H. M. Khalimova, Z. Yu. Khalimova, A. A. Khodjimetov, R. J. Matmurodov, S. M. Umirova
Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Nowadays, one of the urgent problems not only in the field of neurology, but also in the entire field of medicine is diabetes mellitus (DM). Because the complications of this disease bring patients to a state of disability, deeply disrupt the quality of life of patients, and cause socio-economic problems.
Keywords: Diabetes, Diabetic polyneuropathy, Complement, Blood plasma, Dysregulation
Cite this paper: H. M. Khalimova, Z. Yu. Khalimova, A. A. Khodjimetov, R. J. Matmurodov, S. M. Umirova, The Role and Significance of Complement C3 Factor in the Clinical Course of Diabetic Polyneuropathies, American Journal of Medicine and Medical Sciences, Vol. 12 No. 11, 2022, pp. 1166-1170. doi: 10.5923/j.ajmms.20221211.15.
Article Outline
1. Introduction
- The International Diabetes Federation estimates that 425 million people worldwide have diabetes [20], making it the largest global epidemic of the 21st century [24]. 115 million people in China, 73 million in India, and 30 million in the United States have diabetes [26]. In Uzbekistan, in 2015, 170,000 patients with diabetes were registered, and to date, more than 230,000 patients have been registered and monitored. We see that the number of people with diabetes has increased by almost 60,000 in the last 3 years. According to analysis, this figure may exceed 550 million by 2030.
2. The Main Results and Findings
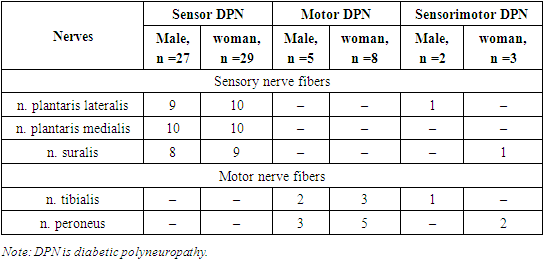
- Diabetic polyneuropathy (DP) is diagnosed in one out of three patients with diabetes and is manifested by a number of negative consequences: neuropathic pain, limitation of daily movement activity, occurrence of trophic disorders and its infectious complications, sleep and mood disorders, quality of life decline Diabetic polyneuropathies are the most common of these complications, which manifest themselves in patients with motor, sensory and vegetative pathologies, as well as increase the disability status of patients and stabilize economic problems.Due to microvascular disorders, the pathogenesis of which is often unclear in QD, complications such as retinopathy, nephropathy and neuropathy also develop, causing serious changes in human health. Patients suffering from diabetes mellitus should not only monitor the concentration of glucose and glycated hemoglobin in the blood, but also perform regular screening with the help of markers that allow to predict the risk of developing microvascular diseases, to understand parallel and separate independent mechanisms [25,19].Currently, there are several types of classification of diabetic polyneuropathies, including the classification approved at the San Antonio conference proposed by the American Academy of Neurology in 1998 [4,11], Levin O.S. in 2006. [5,8,10] and in 2007 Galstyan G.R. Classifications proposed by [1,2] are used.Later, there is increasing interest in the study of complement factors in the process of demyelination in neuropathies. The complement system is considered a key component of innate immunity and plays an important role in inflammation and defense of the body against common pathogens. Activation of the complement system is observed in many metabolic disorders and inflammatory diseases of nerve fibers, including diabetic neuropathies.The complement system is a part of the immune system that protects the body against bacteria and other pathogens in a non-specific manner. The complement system is a collection of membrane-bound proteins that circulate in the blood of humans and animals and participate in tissue damage in autoimmune and other pathological processes, as well as in the protection of the body from infectious diseases [3,7,14].In recent years, the biological and other functions of complements have been studied, such as: phagocytosis, activation of the release of biologically active substances (histamine, serotonin, bradykinin) from fat cells, increase in cell membrane permeability, decrease in blood-vessel tone, positive chemotaxis, opsonization, etc.Components of the complement system make up about 4% of plasma proteins. They are synthesized by hepatocytes, macrophages and neutrophils, and most of them are β-globulins [7]. The complement system is an effector of innate and acquired immunity. It is composed of more than 30 cell membrane and plasma proteins, synthesized mainly in hepatocytes and partly in peripheral nerves [6,9], usually circulating as inactive proproteins.According to the nomenclature adopted by WHO, the complement system is denoted by the symbol S, and its individual components are denoted by the symbols S1, S2, S3, S4, S5, S6, S7, S8, S9.Complement C3 is the most centrally activated component of the complement system in the development of diabetes complications [16,21]. High concentrations of complement C3 are associated with insulin resistance, obesity, dyslipidemia, and diabetes [16,22]. Currently, there is some evidence that complement C3 may play an important role in the development of microvascular diseases [12]. C3d and C5b-9 have been found in the endoneurial microvessels of patients with diabetic neuropathy, and C3 complement overload has been identified in diabetic neuropathies [23]. To date, the increase in the concentration of complement C3 in the plasma of patients, which is associated with a higher risk of developing diabetic microvascular complications, has been little studied. Deposition of immunoglobulins and complement C3 in nerve fibers in diabetic polyneuropathies was first described by Graham and Johnson [15]. Pathological changes in the walls of capillaries, arterioles and venules of nerves lead to the development of early structural abnormalities and decreased perfusion, resulting in ischemic damage to nerve fibers [13]. In diabetes, complement C3 provides a key link between inflammation and thrombosis. Complement C3 interacts with fibrin and causes long-term fibrinolysis in the blood of patients with type 2 diabetes, and as a result of fibrinolysis, neuropathies develop [17,18].A deep analysis of the scientific literature shows that there is very little information on the role and importance of the complement C3 factor in the clinical course of diabetic polyneuropathies. In this context, we found it necessary to study the dysregulation of C3 complement activation in the occurrence and development of diabetic polyneuropathies.
3. The Purpose of Scientific Work
- To study the role and importance of dysregulation of activation of complement component C3 in blood plasma of patients with diabetic polyneuropathy, and to develop early diagnostic criteria.
4. Research Material and Its Methods
- In our research work, we collected the main contingent of patients from the endocrinology department of the multidisciplinary clinic of the Tashkent Medical Academy and the neuroendocrinology department of the Republican Specialized Endocrinology Scientific and Practical Medical Center. During the study, we studied blood plasma samples of 88 patients (men, women) with type 2 diabetes and diabetic neuropathy. We divided the collected patients into 2 groups. The first group consisted of 74 patients with diabetic polyneuropathy (34 men and 40 women), and the second group - the control group - 14 patients (8 men and 6 women) with diabetes, but without diabetic polyneuropathy, and at least one year before the beginning of the research patients who did not suffer from acute infectious or other serious diseases a month ago were selected and their blood plasmas were examined. The total mean age and the total mean duration of the disease were calculated for the patients in the main group and the control group. All patients underwent clinical and neurological examinations. Quality of life levels of patients were assessed based on the EQ 5D-3L questionnaire. Also, blood biochemical analyzes were carried out in patients - determination of blood glucose index and lipid spectrum, as well as blood immuno-enzyme analysis (IFA). In order to determine the activation dysregulation of the complement component C3, blood samples were collected from the patient's vein at 9:00 a.m. on an empty stomach in special vacuum tubes with lithium heparin and immediately placed in a refrigerator. The tubes were centrifuged at 3000 g/min for 10 minutes and blood plasma was separated. The isolated plasma samples were stored in a freezer at -30°C. The results of clinical-neurological and biochemical examinations were compared with the results of electromyographic examination. Statistical methods were used.
5. Research Results
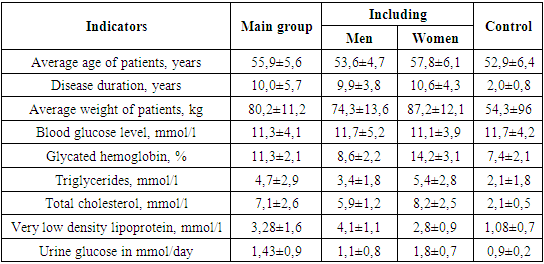
- We found it necessary to study the gender characteristics of diabetic polyneuropathies in the first stages of research. Diabetic polyneuropathies were found in 40 women (54.2%) and 34 cases (45.8%) in men. The average age of patients in the general group was 55.9±5.6 years, and in the control group it was 52.9±6.4 years. The average age of patients in the main group was 53.6±4.7 years in men and 57.8±6.1 years in women. It was found that the duration of the disease was 10.0±5.7 years in the main group, 9.9±3.8 years in women and 10.6±4.3 years in men, and 2.0±0.8 in the control group. The average weight of patients in the general group was 80.2±11.2 kg, 74.3±13.6 kg in men and 87.2±12.1 kg in women, and 54.3±96 in the control group. The blood glucose level of patients in the main group was 11.3±4.1 mmol/l, 11.7±5.2 mmol/l in men and 11.1±3.9 mmol/l in women, and 11.7 in the control group. ±4.2. The amount of blood glucose in men and women in the main group, as well as in patients in the control group, did not differ significantly, r≥0.05. When glycated hemoglobin content was compared, the difference between patients in the main and control groups was significant, 11.3±2.1 and 7.4±2.1, r≤0.05. A significant difference was also observed between men and women in the main group, 8.6±2.2 and 14.2±3.1. When comparing the amount of triglycerides in the blood, the main group of patients was 4.7±2.9 and 2.1±1.8, r≤0.05. These indicators differed in women and men, 3.4±1.8 and 5.4±2.8, r≤0.05. The amount of total cholesterol was 7.1±2.6 in the main group and 2.1±0.5 in the control group, a reliable difference was observed, r≤0.05. 5.9±1.2 and 8.2±2.5 in men and women, the difference is reliable. Very low density lipoprotein was 3.28±1.6 in patients in the main group and 1.08±0.7 in the control group, r≤0.05. 4.1±1.1 and 2.8±0.9 in men and women, the difference is reliable. When the amount of glucose in urine is compared, it is 1.43±0.9 in the main group and 0.9±0.2 in the control group, the difference is reliable. The difference was reliably shown in both men and women. Total cholesterol is normally 0-5.2 mmol/l, triglycerides are normally 0-1.71 mmol/l, very low density lipoproteins in men: 2.25-4.82 mmol/l; in women: 1.92-4.51 mmol/l. The obtained results are shown in Table 1.
|
|
6. Conclusions
- 1. The development of diabetic polyneuropathies has a gender characteristic, it develops faster in women than in men. The average age, weight and duration of the disease of the patient are of great importance.2. Glycated hemoglobin in the blood, total cholesterol, very low density lipoproteins and glucose in the urine can affect the development of diabetic polyneuropathies as risk factors.3. Complement factor S3 is considered to be a factor that enhances demyelination, and its increase in blood serum can be considered as a non-specific marker of the development of diabetic polyneuropathy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML