-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(11): 1162-1165
doi:10.5923/j.ajmms.20221211.14
Received: Nov. 8, 2022; Accepted: Nov. 27, 2022; Published: Nov. 29, 2022

Studying the Possibilities of Individualization of Targeted Therapy Tactics in Colorectal Cancer Patients with Metastatic Liver Lesion
Niyozova Sh. Kh.1, Kamishov S. V. С.2, Balenkov O. Yu.2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Republican Specialized Scientific and Practical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the research was to study the clinical and laboratory factors affecting the efficiency of combination therapy in colorectal cancer patients with metastatic liver lesion. Currently, there are different opinions regarding the treatment tactics and the use of targeted therapy in colorectal cancer (CRC) patients with liver metastases. 75 patients with CRC and metastatic liver lesion were included in the study. All patients received preoperative chemotherapy (PCT) according to the XELOX/FOLFOX4 regimens in combination with the targeted drugs bevacizumab and cetuximab. The combination of PCT with the drug bevacizumab showed the best treatment results. The determination of individual clinical and laboratory risk factors that influenced the long-term treatment results of CRR patients with liver metastases was carried out, which may contribute to the individualization of the tactics of targeted therapy in this category of patients.
Keywords: Bevacizumab, Individual factors, Colorectal cancer, Liver metastases, Chemotherapy, Cetuximab
Cite this paper: Niyozova Sh. Kh., Kamishov S. V. С., Balenkov O. Yu., Studying the Possibilities of Individualization of Targeted Therapy Tactics in Colorectal Cancer Patients with Metastatic Liver Lesion, American Journal of Medicine and Medical Sciences, Vol. 12 No. 11, 2022, pp. 1162-1165. doi: 10.5923/j.ajmms.20221211.14.
1. Introduction
- Colorectal cancer (CRC) is a serious problem of modern oncology due to the steady increase in cases of morbidity and mortality worldwide, including in Uzbekistan. At the same time, CRC is characterized by a high mortality rate, which, on average ranks second in the world among oncological diseases [1]. Hematogenous CRC metastases are distinguished by their predominant spread to the liver as a result of the penetration of tumor cells through the portal system, which reduces the efficiency of surgical intervention. Also, the presence of other combined extrahepatic distant metastases in patients with CRC is not uncommon and reduces the probability of a favorable outcome of the disease [2-4].Treatment standards with cytostatic such as oxaliplatin, irinotecan, as well as 5-fluorouracil (5-FU) and leucovorin have been used in CRC chemotherapy with metastatic liver lesion for a long time. In various randomized studies, it has been shown that the combination of 5-FU with other drugs leads to an increase in the frequency of tumor response to chemotherapy (CT), as well as the median overall survival in patients with CRC and treatment standards include CT regimens such as FOLFOX4 using oxaliplatin; XELOX, which uses capecitabine and oxaliplatin; and also FOLFIRI or XELIRI schemes using capecitabine and irinotecan [5-6]. In recent years, the arsenal of chemotherapeutic effects on the gastrointestinal tract tumors has been supplemented with targeted drugs that allow individualizing therapy. At the same time, there is a modification of the schemes, as well as CT modes in the combined treatment of CRC patients, which contributes to an increase in the overall frequency of tumor response to treatment and patient survival [7-9]. Currently, there is heterogeneous and scarce information in the available literature regarding the efficiency of targeted drugs when used in combination with cytotoxic therapy in the treatment of CRC patients with metastatic liver lesion. In addition, it is not well known yet which target agents may be most effective when used in combination with cytotoxic therapy. It should also be noted that to date there are no uniform standards for the treatment of CRC with distant metastases and no uniform tactics have been developed in the selection of therapy for this category of patients [10-12,4]. The aim of the research was to study the clinical and laboratory factors affecting the efficiency of combination therapy in colorectal cancer patients with metastatic liver lesion.
2. Material and Methods
- The study included 75 colorectal cancer (CRC) patients with metastatic liver lesion who underwent examination and treatment at the Republican Specialized Scientific and Practical Center of Oncology and Radiology for the period from 2015 to 2022. Morphologically confirmed adenocarcinoma of the colon was diagnosed in all patients. All patients were performed chemotherapy (CT) with fluoropyrimidines and oxaliplatin or irinotecan (XELOX/FOLFOX4 regimens) in combination with targeted drugs bevacizumab and cetuximab. CT efficiency was evaluated according to RECIST criteria. Computed tomography and ultrasound examination were performed every 6-8 weeks after the start of treatment according to the RECIST criteria. The severity of the side effects of antiretroviral therapy was estimated according to the NCI CTCAE V.4.0 toxicity scale (2009).In general, there was no significant difference in the proportion of males and females - 52.6% and 47.4%, respectively. Mean age of patients was 62.3±4.9 years, with the largest proportion of patients (52.3%) being over 60 years old. All patients in the study were divided into three groups depending on the chosen scheme:Group 1 – patients who were received standard preoperative polychemotherapy according to XELOX / FOLFOX4 regimens (n=34); Group 2 – patients who were received standard preoperative polychemotherapy according to XELOX / FOLFOX4 + bevacizumab (n=23); Group 3 – patients who were received standard preoperative polychemotherapy according to XELOX / FOLFOX4 + cetuximab (n=18).
3. Results
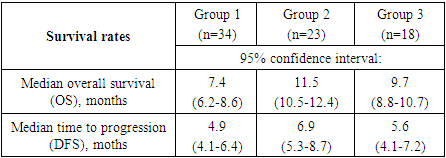
- Rectal adenocarcinoma was histologically verified in all patients. At the same time, in the majority of observations – in 36 patients, in 48.0% of cases, moderately differentiated adenocarcinoma was found. Low-grade adenocarcinoma was detected in 27 (36.0%) patients and 12 (16.0%) patients had highly differentiated adenocarcinoma. The data obtained reflect the fact that most patients had a locally advanced tumor process, in which cells gradually lose their typical morphological characteristics.Ultrasound examination revealed a tumor process in the abdominal cavity in all 75 patients with CRC. In this investigation, the formation of a heterogeneous structure was found in 56 (74.7%) patients, the tumor structure was homogeneous in 19 (25.3%). In 32 (42.7%) patients the tumor had clear contours and in 43 (57.3%) patients the contours of the tumor were fuzzy. During the scan, it was found that 15 (20.0%) patients had a conglomerate of metastatic retroperitoneal lymph nodes, 63 (84.0%) patients had liver metastases. In addition, 15 (20.0%) patients had lesions of paraaortic and/or paracaval lymph nodes. Examination of retroperitoneal organs revealed signs of ureterohydronephrosis in 9 (12.0%) patients.In addition to the standard study, to determine the local prevalence of the process, we performed transrectal sonography in 24 (32.0%) patients with CRC. This technique was carried out to patients with the local spread of the process in order to perform the subsequent most adequate surgical treatment in terms of radicality. Transrectal sonography revealed the presence of a cancerous tumor with a local prevalence of 5.5 to 14 cm in the longitudinal dimension in all patients. In 18 (75.0%) of them during sonography, it was possible to establish the involvement of pararectal fiber in the process, in 20 (83.3%) – lesion of regional lymph nodes.Computed tomography (CT) was performed in all 75 patients with CRC. CT sensitivity was 95.2% in CRC (only in 4.8% of patients, colon tumors were not detected during this examination method). In the presence of a tumor process in patients with CRC, in 78.7% of cases, heterogeneity of the tumor structure was noted, in 85.3% of cases, the fuzziness of the contours of the formation with possible spread to the pararectal zone was established. The results of the studies showed that multiple liver metastases were most common (92.0%), followed by bilobar liver metastases (81.3%). Monolobular liver metastases were diagnosed in 14.7% of patients, single metastases - in 10.7% of patients and solitary liver metastases - in 2.7% of patients with CRC. Our study also showed that the addition of targeted drugs to the treatment regimen significantly improved overall survival and time to disease progression. At the same time, treatment regimens including preoperative CT with XELOX/FOLFOX4 + bevacizumab showed better results, while the targeted agent cetuximab showed lower results (Tab. 1).
|
4. Discussion
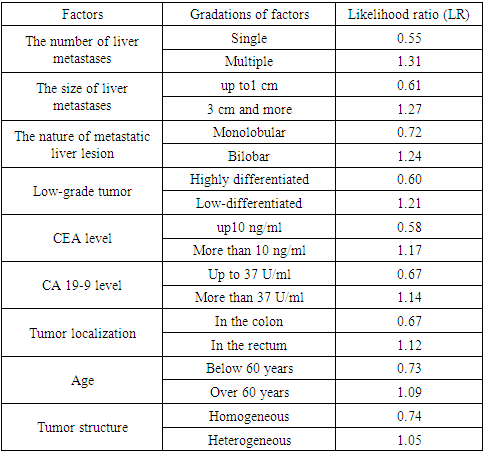
- Currently, there is heterogeneous and scarce information in the available literature regarding the efficiency of targeted agents when used in combination with cytotoxic therapy in the treatment of patients with metastatic liver lesion. It should also be noted that to date there are no uniform standards for the treatment of CRC with distant metastases and no uniform tactics have been developed in the selection of therapy for this category of patients [8-9,4,6]. Based on the conducted studies, we identified individual risk factors that influenced the long-term treatment results of CRC patients with liver metastases based on an integrated evaluation of the data. The likelihood ratio method was used to do this, which allows not only to take into account the degree of consequences probability from the influence of the factor, but also to highlight the most significant risks. Using this method, the overall survival rates (OS) were compared in groups of patients with CRC, in the treatment of which targeted drugs were used, and in the control group of patients (Table 2).
|
5. Conclusions
- Although the treatment results of patients with CRC included in new clinical trials have improved markedly in recent years, existing protocols are limited by the low predictability of chemotherapy efficiency. Therefore, identifying groups of patients who are more likely to respond to the planned therapy than this category of patients as a whole will significantly increase the efficiency of treatment, avoid inappropriate use of certain agents and improve long-term outcomes for patients with metastatic CRC. In the treatment of CRC patients with initially resectable liver metastases, the inclusion of targeted drugs in traditional CT regimens significantly improves the effectiveness of treatment for this type of patients. It was noted that the best results were shown by a treatment regimen including preoperative CT according to the XELOX / FOLFOX4 + bevacizumab regimens, while the targeted drug cetuximab showed lesser results. The most significant factors in our studies reflecting the difference in the results of 5-year survival of patients with CRR with metastatic liver damage were: the presence of multiple liver metastases (LR =1.31), the size of liver metastases more than 3 cm (LR =1.27), bilobar metastatic lesion (LR =1.24), low-differentiated tumor (LR =1.21), повышенный уровень РЭА (LR =1.17), increased level of CA 19-9 (LR =1.14), localization of the tumor in the rectum (LR =1.12), the age of patients older than 60 years (LR =1.09) and the heterogeneous structure of the tumor (LR =1.05). This category of patients is recommended to conduct therapy using targeted drugs.The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML