-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(10): 1056-1058
doi:10.5923/j.ajmms.20221210.10
Received: Sep. 7, 2022; Accepted: Oct. 12, 2022; Published: Oct. 14, 2022

Evaluation of the Efficiency of Urinary Kallidinogenase (Kalgen 0.15 PNA) in Patients with Acute Ischemic Stroke
Avezova S. Yu. 1, Avakov V. E. 2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Stroke is an extremely common disease worldwide. Disability from a stroke ranks first among the causes of primary disability. [2,3] To date, the introduction of new methods of treatment contributes to the success of stroke treatment and greater patient survival. The use of urinary callidinogenase (Kalgen 0.15 PNA) as part of complex therapy for patients with cerebral infarction has an etiopathogenetically effective and rapid recovery of nervous function.
Keywords: Ischemic stroke, Tissue kallikrein (kallidinogenase), MMSE, NIHSS, Rankin, Hemostasis
Cite this paper: Avezova S. Yu. , Avakov V. E. , Evaluation of the Efficiency of Urinary Kallidinogenase (Kalgen 0.15 PNA) in Patients with Acute Ischemic Stroke, American Journal of Medicine and Medical Sciences, Vol. 12 No. 10, 2022, pp. 1056-1058. doi: 10.5923/j.ajmms.20221210.10.
Article Outline
1. Introduction
- Stroke is the second most common cause of death and the leading cause of disability in adults worldwide [2,6] Acute cerebral infarction is caused by a sharp decrease in blood and oxygen supply [1,4] Stroke is a heavy burden for the world due to inadequate traditional treatment available on today. [3] For stroke patients, improved cerebrovascular perfusion, such as thrombolysis, arteriolar expansion and new vessel formation in the ischemic penumbra remains a good option to salvage damaged tissue and alleviate neurological deficits [6,11]. The most severe form of stroke results from occlusion of large vessels of the main branches of the circle of Willis. Treatment strategies currently available in Western countries for large vessel occlusion include rapidly restoring blood flow by removing the problematic blood clot using mechanical or pharmacological means (e.g. tissue plasma activator).Ischemic stroke is caused by hypertension, diabetes, heart disease, age, heredity, and other risk factors. [5,9] This leads to stenosis and occlusion of the lumen of cerebral vessels, as well as to a decrease or interruption of the blood supply to the nerve cells of the brain; thus appears hypoxic-ischemic necrosis. Inflammation at an early stage after cerebral infarction is one of the important mechanisms of neuronal damage in the areas of infarction and penumbra. [2,4]Antithrombotic therapy is an important part of the current treatment of acute ischemic stroke (AIS) [6,7,8] During the onset of AIS, irreversible ischemia and hypoxic necrosis occur in the central region of the infarction due to reduced or intermittent blood supply. However, there are many surviving neurons in the ischemic penumbra of the marginal zone, which can restore their normal function if blood perfusion is restored in a short time. However, due to the short treatment period, most patients miss the best time for thrombolysis during treatment. [10] Angiogenesis plays a key role in the process of nerve repair, which mainly occurs in the border zone of ischemia. When blood flow in the local brain tissue is blocked, blood can enter the ischemic region through other collateral branches. The newly formed blood vessels can then promote neurorecovery processes, including neurogenesis and synaptogenesis, thereby improving functional recovery. [12]Human urinary kallidinogenase (HUK), which is up-regulated via the kallikrein-kinin (KKS) system, catalyzing the hydrolysis of low molecular weight kininogens to vasoactive kinins, and thereby activates the bradykinin B1 and B2 receptors (B1R and B2R) and has a number of biological effects. Of these, activated B2 receptor can promote angiogenesis through Akt-GSK-3β-VEGF-VEGFR-2 and Akt-eNOS-NO signaling pathways. The protective mechanisms of kallikrein in ischemic brain damage include anti-inflammatory and anti-apoptotic effects, as well as stimulation of angiogenesis and neurogenesis in hind limb ischemia, myocardial infarction and renal ischemia. [14]Objective: To improve the results of treatment of patients with acute ischemic stroke.
2. Clinical Materials and Research Methods
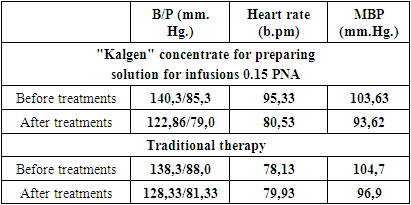
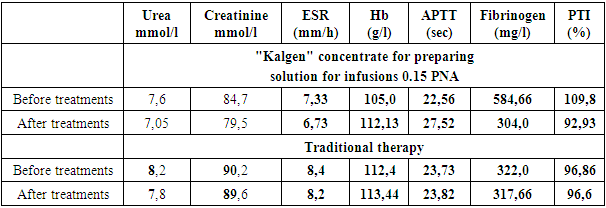
- We examined 24 patients with acute ischemic stroke (18 men and 6 women) in the intensive care unit of the clinic of the Urgench branch of the TMA and in the emergency neurology department of emergency hospital, whose average age was 58.1 ± 4.4 years. We divided all patients into 2 groups: the control group, which included 12 patients, received standard therapy (antioxidants, neuroprotectors, detoxifications, anticoagulants (low molecular weight heparins), sedative and symptomatic therapy) and the study group, which included the remaining 12 who, in addition to the indicated therapy received Kalgen 0.15 PNA (urinary callidinogenase) once a day, diluted with saline intravenously, drip, slowly.Both groups were randomized by us according to gender and age characteristics, the nature of the standard examination and according to MSCT data.All patients underwent clinical and biochemical studies, computed tomography (CT), during therapy they monitored blood pressure (BP), mean arterial pressure MAP (according to the formula: SBP, mm Hg = (Syst. BP + 2 Diast. BP): 3), central venous pressure (CVP), blood glucose, thermometry and saturation of venous blood. We assessed the neurological status using the MMSE, NIHSS, Rankin.In addition to general clinical methods of blood and urine analysis, coagulogram parameters, biochemical parameters of blood, markers of kidney function (urea, creatinine) were monitored in all patients of the study and control groups.The length of stay of patients in the ICU and in the multidisciplinary clinic of the Urgench branch of TMA as a whole was studied.Study design: single center prospective study.
3. Results of Own Research
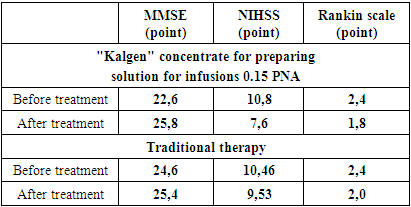
- Average data for predicting the severity of functional disorders (MMSE scale, NIHSS, Rankin Scale) are shown in the table
|
|
|
4. Conclusions
- 1. Kalgen 0.15 PNA (urinary callidinogenase) etiopathogenetically provides an effective and rapid recovery of nervous function in cerebral infarction.2. Kalgen in the complex therapy of patients with ischemic stroke, reducing plasma fibrinogen and PTI by 48.1% and 15.4%, respectively, and increasing the APTT time by 21.9%, significantly improves hemostasis.3. Kalgen (urinary callidinogenase) is well tolerated by patients and does not cause any complications.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML