Saydimurad I. Ismailov 1, Rustam A. Sadikov 2, Axmadjan S. Babajanov 3, Aleksey O. Soy 4, Jamshid N. Mardonov 5
1Director, Doctor of Medical Sciences, Republican Specialized Scientific and Practical Medical Center for Surgery Named after Academician V. Vakhidov, Tashkent, Uzbekistan
2Head of the Training Center, Doctor of Medical Sciences, Republican Specialized Scientific and Practical Medical Center for Surgery Named after Academician V. Vakhidov, Tashkent, Uzbekistan
3Associate Professor of the Department of Surgery, Samarkand Medical Institute
4Senior Researcher, Department of Surgery of the Oesophagus and Stomach, Candidate of Medical Sciences, Republican Specialized Scientific and Practical Medical Center for Surgery named after academician V. Vakhidov, Tashkent, Uzbekistan
5Head of Pathological Anatomy, Candidate of Medical Sciences, Republican Specialized Scientific and Practical Medical Center for Surgery named after academician V. Vakhidov, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article discusses morphological assessment of laser efficiency in the treatment of oesophagus injury. Of course, one of the main diagnostic criteria today is the performance of various endoscopic examinations before the operation, which is carried out in the main direction of surgery. With the development of endoscopic techniques, it became possible to perform various palliative or reconstructive endosurgical operations using them [6,7,12,14,15].
Keywords:
Morphological assessment, Laser efficiency, Treatment of oesophagus injury
Cite this paper: Saydimurad I. Ismailov , Rustam A. Sadikov , Axmadjan S. Babajanov , Aleksey O. Soy , Jamshid N. Mardonov , Morphological Assessment of Laser Efficiency in the Treatment of Oesophagus Injury, American Journal of Medicine and Medical Sciences, Vol. 12 No. 9, 2022, pp. 1006-1012. doi: 10.5923/j.ajmms.20221209.33.
1. Introduction
While the development of surgical or diagnostic endoscopic procedures, in turn, provides additional opportunities, at the same time, with various technical complexities of the practice, various complications also arise. One such complication is traumatic injury to the ooesophagus. It should be added that traumatic damage to the ooesophagus can develop as a complication of various other diseases or polytrauma, as well as under the influence of coarse food masses. As a result, depending on which anatomical site is affected, there may be a violation of the integrity of the wall - perforations of different sizes, resulting in the release of various foreign bodies or an infectious agent into the mediastinum or abdominal cavity. This can eventually lead to various infectious complications.The anatomical feature of the oesophagus is that it simultaneously comes into contact with both the mediastinal region and the abdominal region [1,2,8].Among the complex operational nosologies performed in surgical gastroenterology, one of the most common are operations performed on the ooesophagus.Anatomically and physiologically, the oesophagus is an important organ involved in the movement of food masses during digestion. Currently, there is no clear surgical tactics for damage to the oesophagus. For this reason, the authors have now proposed several different tactics. These methods also have not yet fully found their clinical solution [5-8].In case of damage to the oesophagus, the primary task of the surgeon must necessarily include measures to restore the integrity of the organ and restore the morphofunctional state in the affected areas [5-8].Based on this, we set the task to conduct a morphological comparative assessment of damage to the oesophagus in the proposed experimental model and changes that occur when exposed to NILI.Precise conclusions about the objectives of the study can be obtained mainly by morphological evaluation under experimental conditions. To this end, we examined the results by morphological evaluation of the experimental results.
2. Material and Methods
To do this, an upper midline laparotomy is performed in experimental animals under general anesthesia with isoflurane vapor. After traction of the left lobe of the liver up and to the right, the cardial section of the stomach is mobilized by dissecting the ligaments. The oesophagus is mobilized in the abdominal region and taken on a holder. Its diameter is up to 2-3 mm. Opening the lumen of the oesophagus by dissecting the wall in the transverse direction using microsurgical scissors. For control, a catheter or a metal probe with a diameter of 1x1 mm is inserted into the lumen of the oesophagus and passed into the lumen of the stomach. Next, the oesophagus is removed from the holder. A suspension of microbial culture in the amount of 0.5 ml of microbial suspension is introduced into the area of the esophageal defect and the subhepatic space. The surgical wound is sutured in layers with nylon atraumatic sutures.Experimental studies were carried out on white outbred male rats with an average weight of 210–260 g.At the same time, biomaterials obtained from experimental animals after surgery were examined on days 3, 7, and 10.The obtained biomaterials were fixed in 10% formalin solution in phosphate buffer. Paraffin sections were stained with hematoxylin and eosin.Light-optical micrographs were taken on a DN-300M microscope coupled with a digital camera and a computer.All microphotographs were processed and saved on a computer using Microsoft-"Windows 10 pro" application programs.
3. Results
On the 3rd day after the formation of damage to the oesophagus in the control group, various alterative inflammatory processes predominated in almost all layers. There are violations of the histoarchitectonic order in all layers, necrotic changes in different histological layers, diffuse lymphocytic-leukocyte infiltration in the subcutaneous layer, chaotic changes in the connective tissue layer, thickening of the vascular wall, vascular dilation, fullness (stasis), diapedesis of erythrocytes around the vessels, oedema throughout layer. In the outer serious (adventitial) layer of the oesophagus, infiltration with neutrophilic-lymphocytic foci is observed (Figures 1-5).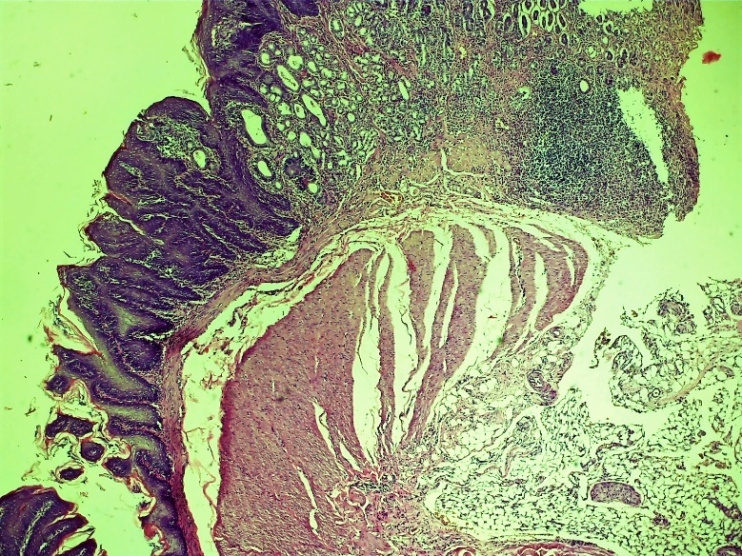 | Figure 1. Esophageal wound area. Alterative disturbance of the order of histoarchitectonic layers. Oedema of tissue layers, hemorrhages of various sizes, diffuse lymphocytic-leukocytic focal infiltration. Control group 3 days. G-E. CM 10x4 |
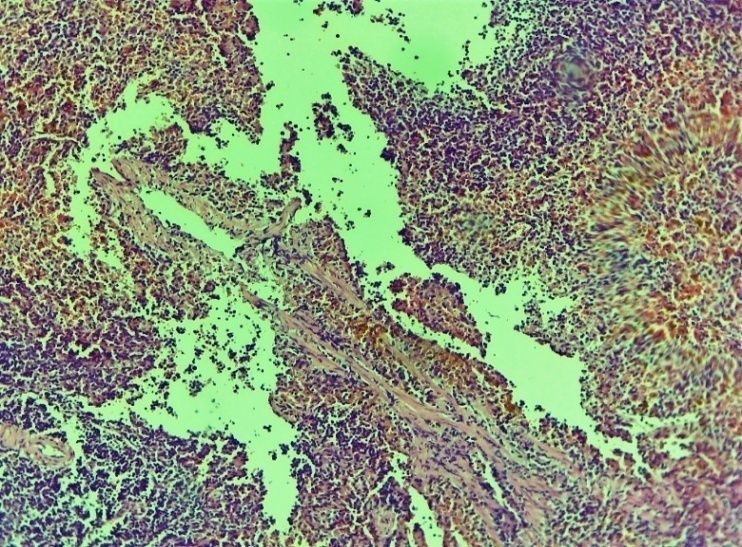 | Figure 2. Area of damage to the oesophagus. Necrotic changes in the histological layers, diapedesis of erythrocytes around the vessel, oedema and neutrophil-lymphocytic focal infiltration throughout the layer. Control group 3 days. G-E. CM 10x4 |
 | Figure 3. Cardioesophageal zone of injury to the oesophagus. Alternative violation of the order and integrity of the histoarchitectonic layers. Tissue oedema, hemorrhages of various sizes, chaotic (incorrect) changes in the connective tissue layer, thickening of the vessel wall, dilatation of the vessels in various forms, stasis. Control group 3 days. G-E. CM. 10x4 |
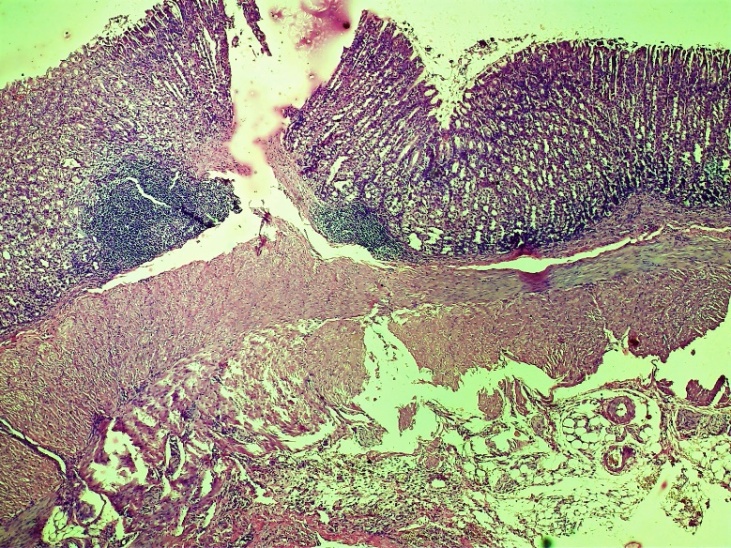
 | Figure 4. Esophageal wound area. Violations of the histoarchitectonic order in all layers, necrotic changes at different levels of the histological layer. Diffuse lymphocytic-leukocytic focal infiltration extends to the entire layer. Control group 3 days. G-E SM 10x4 |
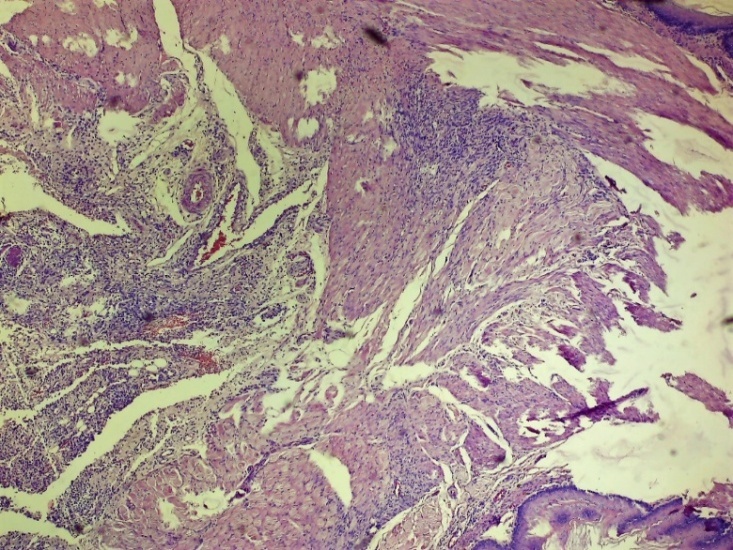 | Figure 5. Area of damage to the oesophagus. Chaotic changes in the connective tissue layer, thickening of the vessel wall, dilatation of vessels of various shapes, completeness (stasis), diapedesis of erythrocytes around the vessel and swelling of the entire layer. Control group 3 days. G-E. CM 10x4 |
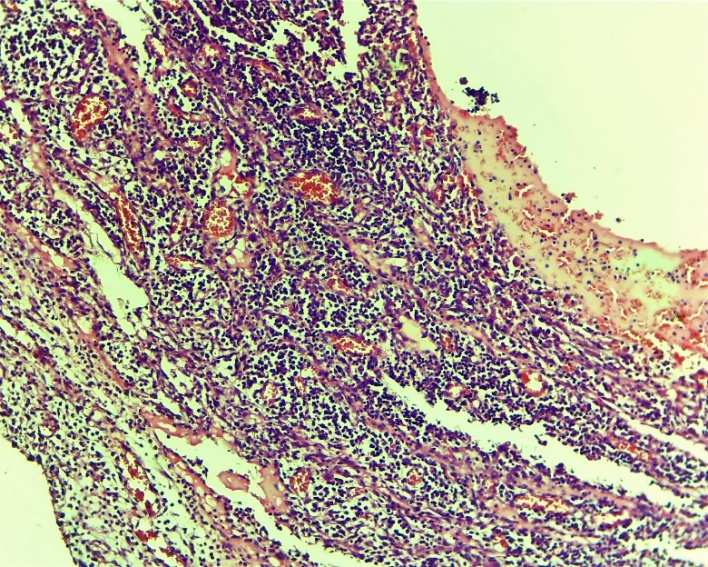
At this time, the exudative-proliferative process of inflammation prevailed in the experimental group. At the same time, oedema of the subcutaneous areas, infiltration with lymphocytic foci were very rarely observed. The blood vessels are full (Figures 6, 7 and 8).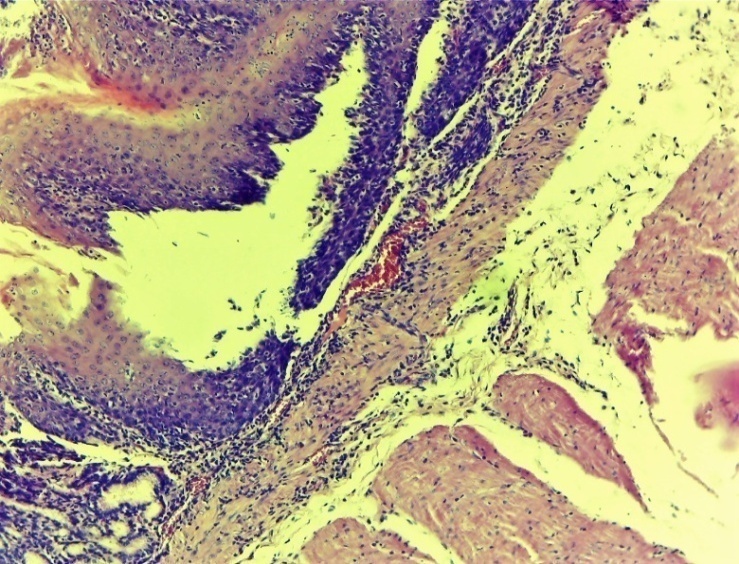 | Figure 6. Esophageal wound area. Oedema of the submucosal layer, infiltration of a small number of lymphocytic foci. Experienced group. 3 days. MR SM. 10x4 |
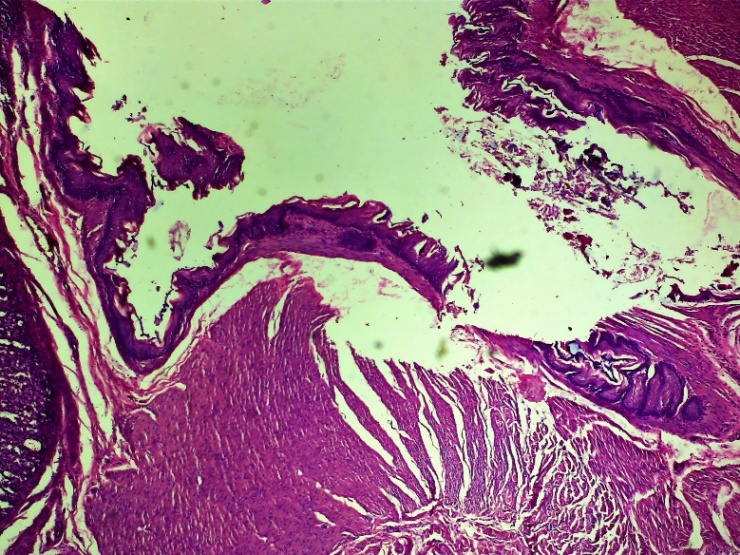 | Figure 7. Histoarchitectonic layers of the wound area of the oesophagus. Fibroblasts have formed. The blood vessels are full. Experienced group. 3 days. G-E. CM 10x4 |
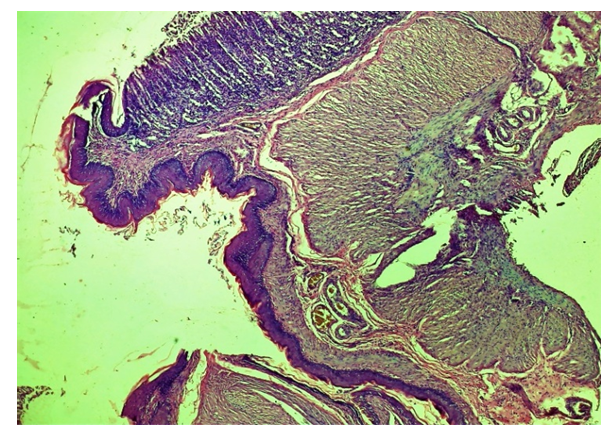 | Figure 8. Cardiac area of injury to the oesophagus. Cell-free areas - areas of tissue oedema, extravascular erythrocyte, lymphocytic-macrophage focal infiltrates and chaotic primary fibroblasts. Areas of neovascularization appeared. Experienced group. 3 days. G-E. CM 10x2 |
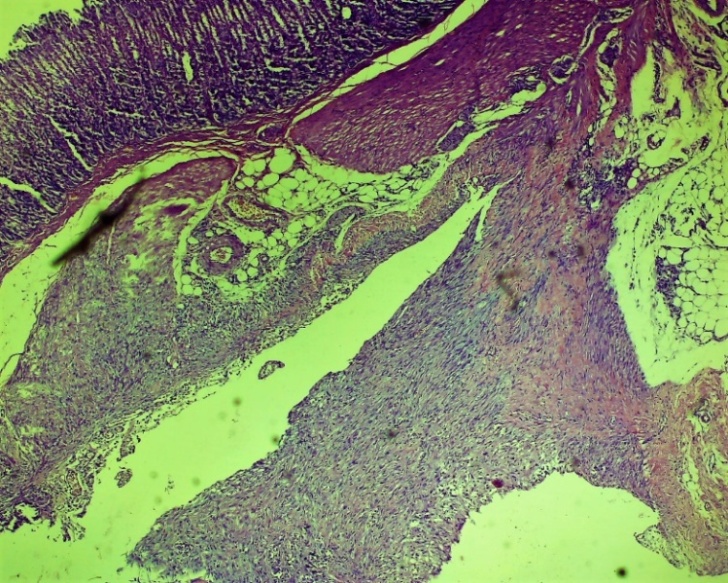
On the other hand, the above changes form specific cell-free areas - tissue tumors, extravascular erythrocytes, lymphocytic-macrophage foci of infiltration and chaotic primary fibroblastic areas. Fibroblasts formed in the damaged area of the oesophagus. Also, according to the laws of regeneration, the formation of new vessels (neovascularization) began to appear in the areas of fibroblasts (Fig. 7 and 8).On the 7th day of the experiment in the control group, necrobiotic processes prevailed in the process of exudative-proliferative inflammation in the mucous and submucosal layers. Necrotic changes in the mucous membrane, focal infiltration by macrophage lymphocytes in the subcutaneous layer, uneven expansion of the connective tissue layer, thickening of the vascular wall, dilatation and stasis in various forms, diapedesis of erythrocytes around the vessel, oedema throughout the vessel. In non-cellular areas, predominantly erythrocyte and lymphocytic-macrophage foci of infiltration predominate. In the affected serious (adventitia) layer of the oesophagus, neutrophil-lymphocytic focal infiltration prevailed. The formation of the first chaotic fibroblasts occurred in the wound area. (Figures 9, 10, 11 and 12).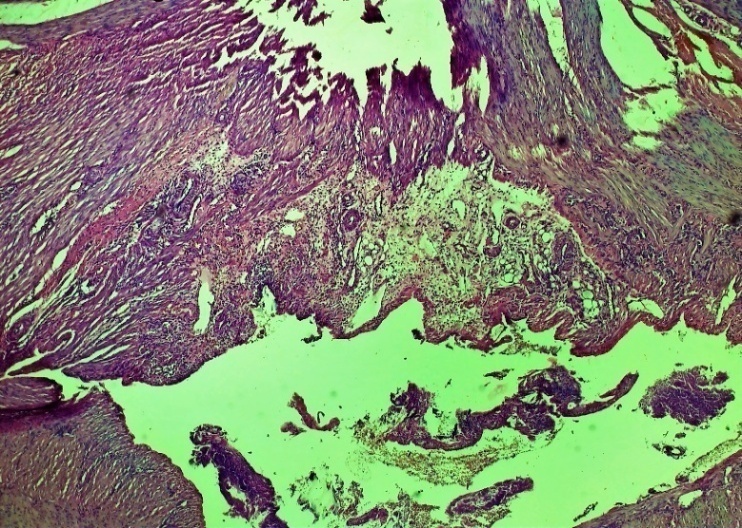 | Figure 9. Esophageal wound area. Necrotic changes in the mucous membrane, focal infiltration of lymphocytes-macrophages in the submucosal layer, uneven growth of the connective tissue layer. Control group 7 days. G-E. CM 10x4 |
 | Figure 10. Layers of the damaged area of the oesophagus. Uneven expansion of the connective tissue layer, thickening of the vessel wall, dilatation and stasis of various shapes, oedema throughout the layer. Fibroblasts formed in areas of the submucosa and various intermittent muscle layers. Control group 7 days. G-E. CM 10x4 |
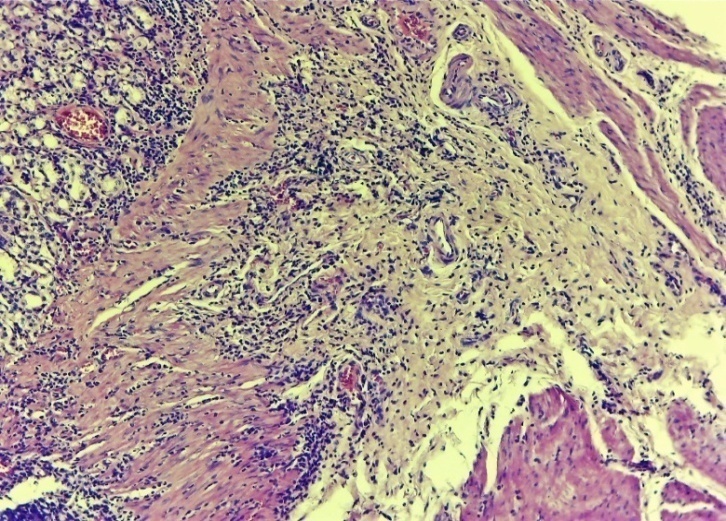
 | Figure 11. Layers of the oesophagus. Chaotic growths of the connective tissue layer, fibrosis in areas of the muscle layer. Tissue oedema. Infiltration by neutrophilic-lymphocytic foci in the outer serious layer. Control group 7 days. G-E. CM 10x4 |
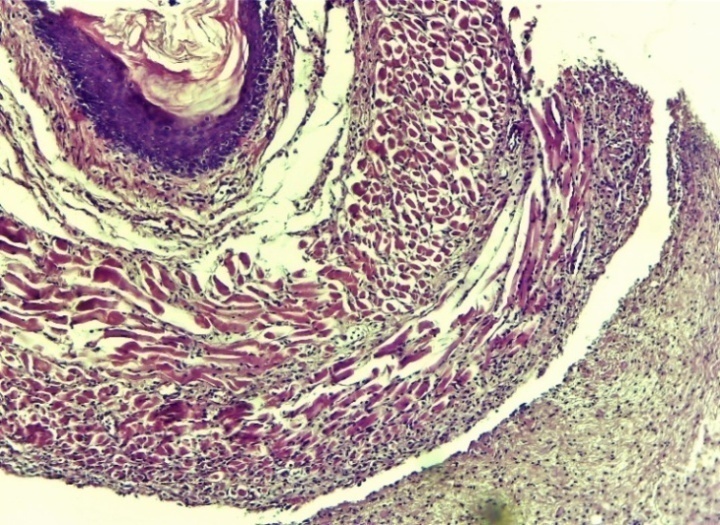
 | Figure 12. Zone of cardioesophageal damage to the oesophagus. In non-cellular areas, predominantly erythrocyte and lymphocytic-macrophage foci of infiltration predominate. Uneven expansion of the connective tissue layer. Infiltration by neutrophilic-lymphocytic foci in the outer serious layer. Fibrotic changes and swelling in areas of the muscle layer. Control group 7 days. G-E. CM 10x4 |
In the experimental group, on the 7th day, the proliferative process of inflammation prevailed. In the existing damaged (injured areas) layers, thin fibroblasts are clearly formed, reparative regeneration prevails. Infiltration of histocytes and macrophages is observed in different layers (Fig. 13, 14 and 15). This, in turn, served as the basis for the formation of new epithelial cells in the damaged (dystrophically and necrotically) epithelial layer [4]. These symptoms are mainly observed on the 7th day (Fig. 13, 14 and 15), and more precisely on the 10th day (Fig. 16-19).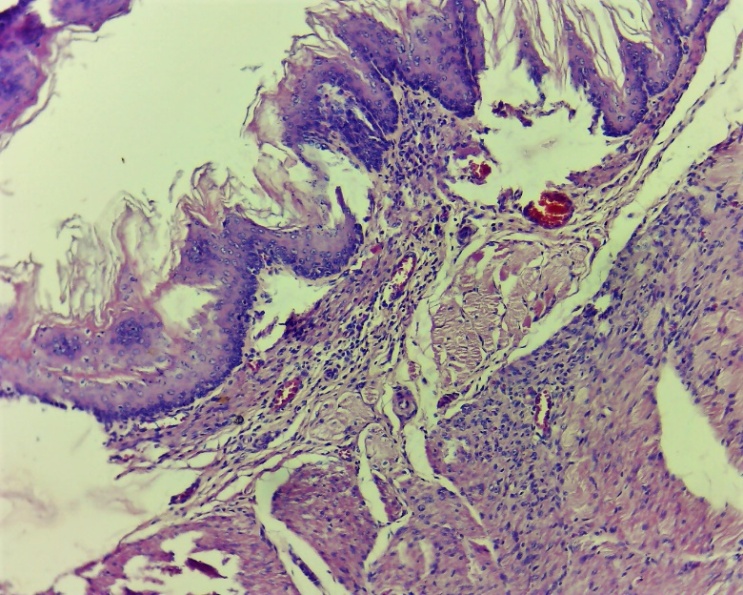 | Figure 13. Layers of the wound area of the oesophagus. Thin fibroblasts formed in the damaged layers. Diffuse infiltration by histocytes and macrophages. Experimental group 7 day. G-E. CM 10x4 |
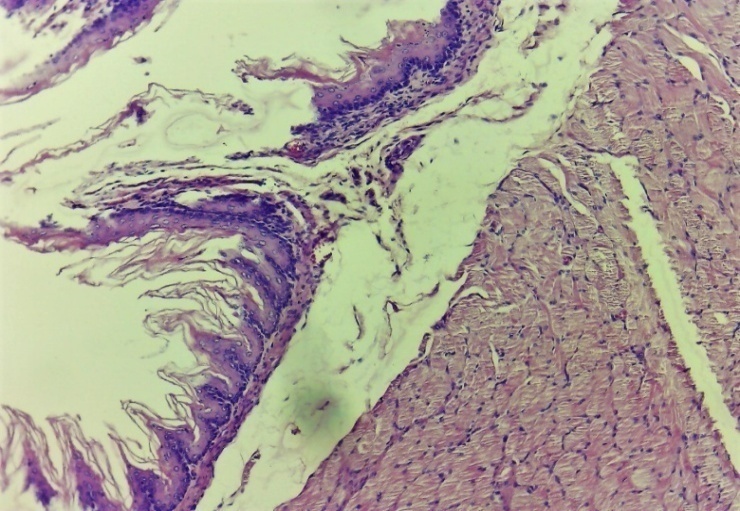 | Figure 14. Signs of proliferative dominance in the area of damage to the oesophagus. In the damaged (dystrophic and necrotic) epithelial layer, new epithelial cells began to form. Infiltration of histocytes and macrophages in different layers. Experimental group 7 day. G-E. CM 10x4 |
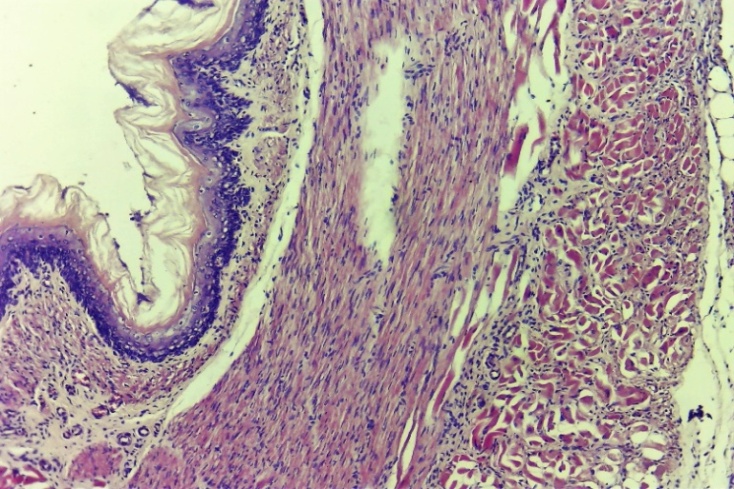 | Figure 15. Histological layers of the oesophagus. In the damaged (dystrophic and necrotic) epithelial layer, new epithelial cells began to form. The predominance of reparative regeneration, in which thin fibroblasts are clearly formed. Experimental group 7 day. G-E. CM 10x4 |
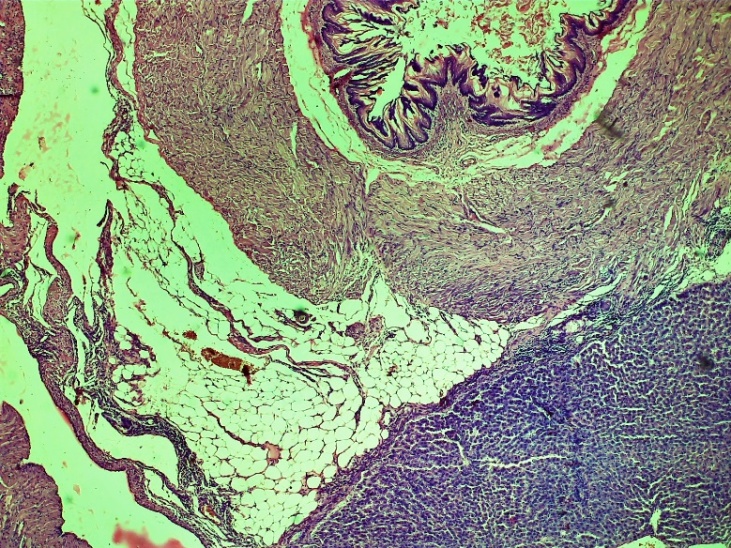 | Figure 16. The area of the lesion of the oesophagus with adhesive areas to the liver. Fibroblasts form in the affected submucosa and muscle layer. Among the affected areas, lymphocytic-macrophage inflammatory infiltration was observed. Fibroblasts are rough, accompanied by swelling. The restoration of the mucous and submucosal layers is nearing completion. Control group 10 days. G-E. CM 10x4 |
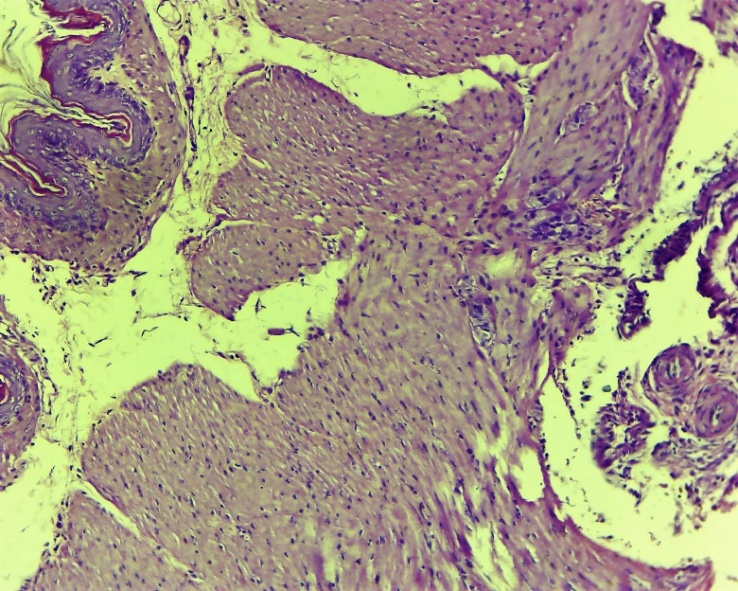 | Figure 17. Esophageal wound area. The restoration of the mucous and submucosal layers is nearing completion. Fibroblasts were formed in this layer. Fibroblasts are rough, accompanied by swelling. Lymphocytic-macrophage inflammatory infiltration was observed. Control group 10 days. G-E. CM 10x4 |
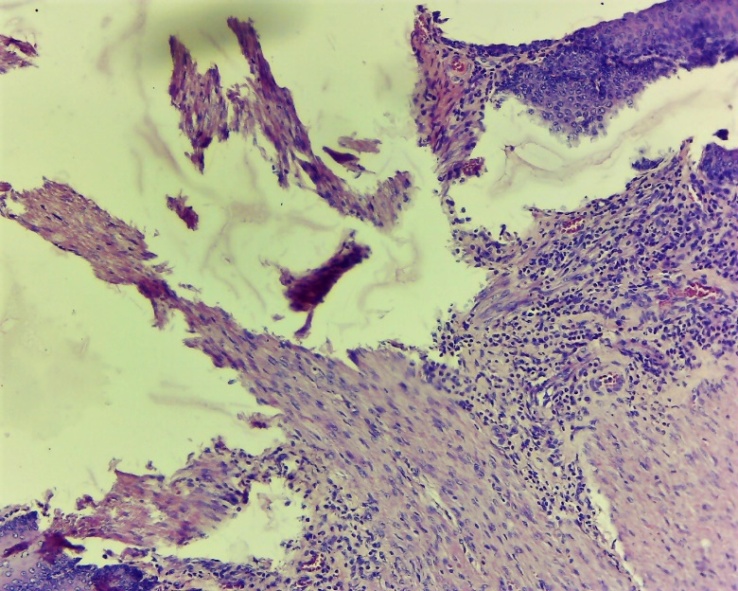 | Figure 18. The affected layers of the oesophagus. Development of regeneration of coarse connective tissue in the wound area, lymphocytic-macrophage inflammatory infiltration among the affected areas. Experienced group. Day 10 G-E. CM 10x4 |
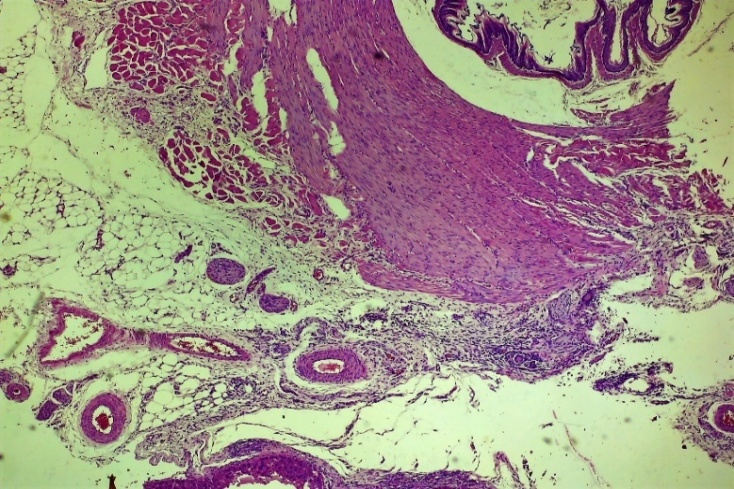 | Figure 19. Layers of the oesophagus. Normalization of mucosal and submucosal histoarchitectonics. Development of regeneration of coarse connective tissue, focal lymphocytic-macrophage inflammatory infiltration. vascular fullness. Experienced group. Day 10 G-E. CM 10x4 |
In the control group, by the 10th day, the initial proliferative processes began to predominate, but the predominance of exudative changes in the process remained. In the area of the wound, regeneration of coarse connective tissue formations was observed, lymphocytic-macrophage inflammatory infiltration was observed in almost all areas, especially among the affected areas. Various alliances were formed with neighboring members. It has been shown that fibroblasts are formed in the affected layers. But here, in contrast to the experimental group, fibroblasts were located chaotically, unevenly and were accompanied by swelling. The restoration of the mucosal and submucosal layers is nearing completion (Figures 16 and 17).On the 10th day of the experiment, the layers of the oesophagus in the affected areas began to completely regenerate in the experimental group (Fig. 20-23). In the area of traumatic injury, growth of soft fibrous connective tissue is observed. The acceleration of cell differentiation and transformation led to the regeneration of the epithelial layer and was manifested by histocytic oedema around different layers (Fig. 20). The formation of new vessels (neovascularization) is accelerated, the vessels are filled. In a word, it was found that the entire layer was again restored to its morphophysiological state (Fig. 20 and 21).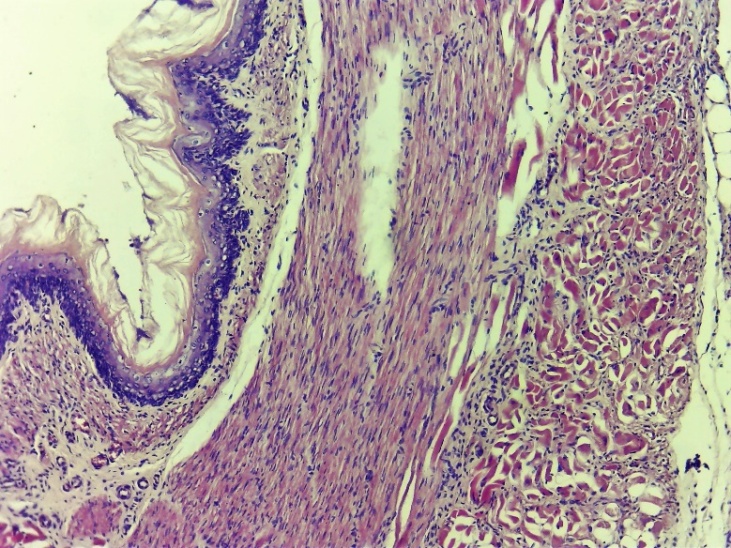 | Figure 20. Complete restructuring of the histological layers in the affected area of the oesophagus. There was regeneration of the epithelial layer and some histocytic tumors around different layers. The formation of new vessels is accelerated, the vessels are filled. Control group 10 days. G-E. CM 10x4 |
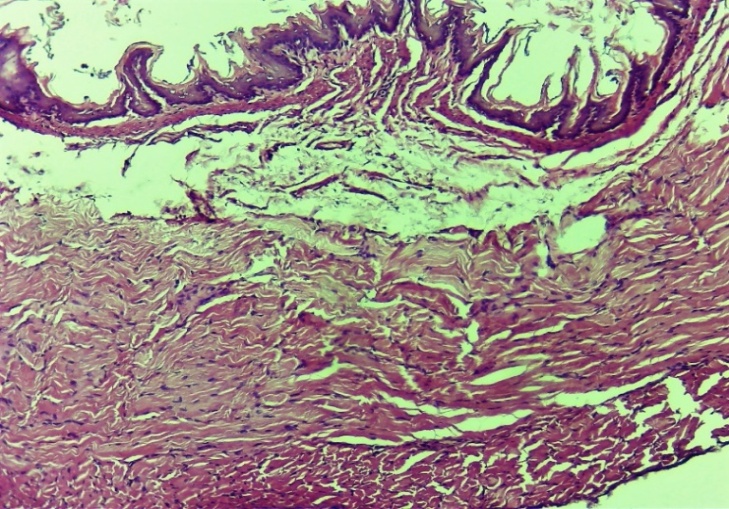 | Figure 21. Complete histoarchitectonic layering of the esophageal lesion. Control group 10 days. G-E. CM 10x4 |
 | Figure 22. Completely regenerated layers of the oesophagus. Very few fine-grained connective tissue elements are observed in the wound area. Formed new blood vessels (neovascularization). Experimental group 10 days. G-E. CM 10x4 |
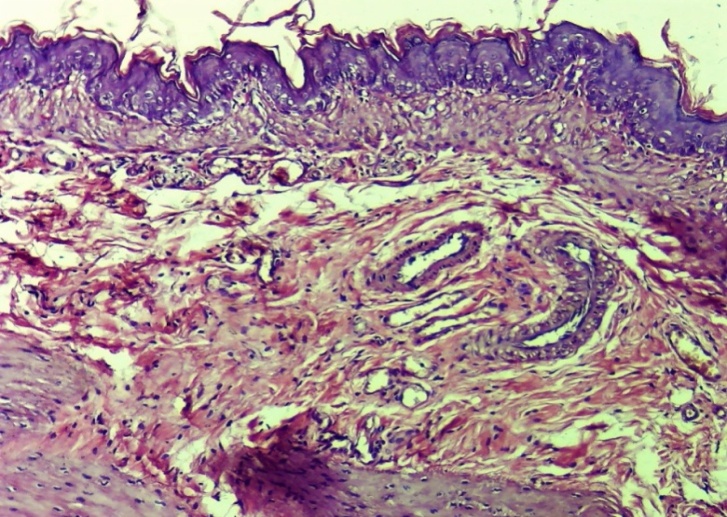 | Figure 23. Histoarchitectonic altered layers of the oesophagus. In areas of the affected muscle layer, elements of soft fibrous connective tissue are observed. Formed new blood vessels (neovascularization). Vessels are full. Experimental group 10 days. G-E. CM 10x4 |
4. Discussions
Currently, lasers are used in various fields of medicine. Of these, the most important phototherapeutic ones are, of course, low-intensity lasers [3,9].The modern application of low-intensity laser radiation in various areas of surgery quickly normalizes the process in these pathological zones and normalizes their morphophysiological balance [10,11].Laser beams of various wavelengths and radiation modes, which are now known, are widely used in surgery. They have the ability to reduce various pathological processes depending on the wavelength and intensity. Among them, the use of UV bands in surgery has a number of advantages. It is known from recent studies that UV irradiation indirectly affects all stages of the inflammatory process, especially the alteration and exudative processes, reduces the inflammatory response, reduces swelling in all tissue layers, and stimulates regeneration processes [12].Another important aspect is that NILI irradiation stimulates almost all stages of the process, having a complex effect on the stage of inflammation. This, in turn, reduces the process of scarring due to the weakening of the inflammatory processes that can occur after the pathological condition of various traumatic injuries of the oesophagus. Another specific aspect is that NILI directly and indirectly stimulates microcirculation. From the laws of the theory of reparative regeneration, it is known that the basis of any pathological inflammation is a violation of microcirculation. If this is restored, regeneration will accelerate and the wound will heal [10,11].Our results also revealed the predominance of wound healing, infiltrative processes and adhesions that can occur with neighboring organs in the experimental group.
5. Conclusions
Based on the foregoing, these features and changes were confirmed by a comparative study of the results obtained as a result of experimental morphological studies conducted in our study.Instead of the last word, in short, the combined use of PILS in traumatic injuries of the oesophagus manifests itself in the following interrelated morphophysiological changes:1) Stimulates all stages of the process of reparative regeneration in the area of damage to the oesophagus.2) Prevents the development of primary and secondary infectious agents in traumatic injuries as a result of direct stimulation of the local immune system and indirect stimulation of microcirculation.3) Causes tissue regeneration as a result of stimulation of cell differentiation and transformation, which is an important link in the regeneration process.In general, all this together prevents early or late reactions that can occur at the end of various anastomotic or traumatic wound injuries performed in esophageal surgery.
References
| [1] | Bibikova A. A. et al. Features of the anatomical structure of the diaphragmatic-esophageal segment // Tver Medical Journal. – 2020. – no. 3. - S. 20-24. |
| [2] | Bilic G., Zigalova E. Human Anatomy. – Literes, 2022. |
| [3] | Gafurov S. D., Katakhonov Sh. M., Kholmonov M. M. Features of the use of lasers in medicine // European science. – 2019. – no. 3 (45) |
| [4] | Zakharova N. M. et al. Physiological significance of proliferative and alterative processes // Successes of physiological sciences. - 2013. - T. 44. - No. 3. - S. 33-53. |
| [5] | Zubarev P. et al. (ed.). Surgical diseases of the oesophagus and cardia. – Literes, 2022. |
| [6] | Ivanov AI et al. Endoscopic treatment of esophageal perforations and esophageal anastomosis leaks // Practical Medicine. - 2019. - T. 17. - No. 6-2. - S. 74-80. |
| [7] | Ivanov A. I., Popov V. A., Burmistrov M. V. Endoscopic stenting for esophageal perforations // Endoskopicheskaya Khirurgia. - 2021. - T. 27. - No. 3. |
| [8] | Kiselevsky Yu. et al. Topographic anatomy and operative surgery. – Literes, 2022. |
| [9] | Konchugova T. V. et al. Influence of physical factors on regeneration processes // Questions of balneology, physiotherapy and exercise therapy. - 2021. - T. 98. - No. 3-2. – S. 93-93. |
| [10] | Poddubnaya OA Low-intensity laser therapy in clinical practice (part No. 1) // Bulletin of restorative medicine. – 2020. – no. 6 (100). |
| [11] | Pushkar Yu. Yu., Badikov DV, Pykhteev VS Study of the influence of low-intensity laser radiation on the dynamics of the course of the wound process // Scientific Bulletin of Public Health of the Kuban. – 2020. – no. 5. - S. 11-20. |
| [12] | Hamdan Ya. et al. Study of the influence of the duration of laser pulses in the ultraviolet range of the spectrum on cells // 65th International Scientific Conference of the Astrakhan State Technical University. - 2021. - S. 694-698. |
| [13] | Chernousov A., Khorobrykh T., Bogopolsky P. Surgery for peptic ulcer of the stomach and duodenum. – Literes, 2022. |
| [14] | Shuklin G. O. et al. Acute mediastinitis as a complication of esophageal perforation // International Student Scientific Bulletin. – 2019. – no. 3. - S. 19-19. |
| [15] | Bustos R. et al. Robotic hepaticojejunostomy: surgical technique and risk factor analysis for anastomotic leak and stenosis // HPB. - 2020. - T. 22. - No. 10. - S. 1442-1449. |
| [16] | McCarty TR, Thompson CC Lumen Apposition: A Changing Landscape in Therapeutic Endoscopy //Digestive Diseases and Sciences. - 2022. - S. 1-14. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

















