Ozoda Alibekovna Mirzabekova
Assistant, Tashkent Medical Academy, Uzbekistan
Correspondence to: Ozoda Alibekovna Mirzabekova, Assistant, Tashkent Medical Academy, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this scientific study, morphological and morphometric indicators of hyaline membrane disease in premature infants were studied. The material was obtained from the results of clinical–anamnestic analysis of preterm infants at 22–37 weeks and lung tissue. The results show that the immaturity of lung tissue, circulatory disorders and damage to the alveolar and bronchial epithelium play an important role in the development of hyaline membrane disease in the lungs of premature infants. The results of morphometric studies showed that only 1/3 of the lungs of infants born at 22–27 weeks had air in the alveoli, the thickness of the alveolar interstitial tissue, the epithelium of the alveoli was relatively large, and the resulting hyaline membranes were composed of relatively thin fibers. At 28–32 and 33–37 weeks, the dying groups observed an expansion of the area occupied by airborne alveoli in the infant’s lungs, thinning of the interstitial tissue, and thickening of the hyaline membranes compared to the previous group.
Keywords:
Infant, Premature birth, Lung, Alveoli, Hyaline membrane, Morphology
Cite this paper: Ozoda Alibekovna Mirzabekova, Morphological and Morphometric Characteristics of Gialin Membranal Disease in Baby Birth, American Journal of Medicine and Medical Sciences, Vol. 12 No. 9, 2022, pp. 987-990. doi: 10.5923/j.ajmms.20221209.28.
1. Relevance of the Topic
According to the criteria adopted by the World Health Organization in 1970, the lower limit of live birth is the 22nd week of gestation, in which the fetus weighs 500 grams and has a body length of 25 cm [3]. As a result, the number of premature births has not decreased despite the measures taken to strengthen antenatal prophylaxis to improve pregnancy [1,2]. In the neonatal intensive care unit, a new type of contingent, i.e. a group of infants with extremely low body weight, has emerged. It is well known that the lower the body weight, the higher the incidence of disease, including an increased incidence of hyaline membrane disease (GMD) [5]. However, GMD antenatal prophylaxis has been developed, but morbidity and mortality remain high [6].In addition, modern technology measures, including: high–intensity artificial ventilation of the lungs, the use of exogenous surfactants have led to a change in GMD symptoms. To date, invasive and non–invasive methods are used in the treatment of GMD, including the increase of positive pressure in the respiratory tract, the introduction of endogenous surfactants [7,8]. However, studies devoted to morphological and morphometric examination of the lungs of those who died from GMD are considered to be non–existent.The purpose of this study was to determine the specificity of the morphological changes that develop as a result of GMD in the lungs of premature infants.
2. Materials and Methods
As material, autopsy data were obtained from 52 premature infants of various degrees who died of pulmonary insufficiency. Initially, the infants’ medical history and autopsy protocol were analyzed. Premature infants were divided into the following groups by gestational weeks: group 1 – 12 at 22–27 weeks (23.1%); group 2 – 18 at 28–32 weeks (34.6%); group 3 – 22 infants born at 33–37 weeks (42.3%) and died of pulmonary insufficiency (Table 1). Of these, group 1, i.e., infants who were deeply premature at 22–27 weeks, had very low body weight, and died around 1 hour after birth, were taken as the control group. The goal was to identify the first morphological changes in the lungs of this group of infants, leading to the formation of GMD. The main cause of death in this group was postnatal asphyxia. In all cases, the presence of prenatal and intranatal risk factors by the mother, the presence of pathologies by the placenta and umbilical cord were detected. The anthropometric indicators of the infants examined are given in Table 1.Table 1. Anthropometric indicators of premature infants, М±m
 |
| |
|
The results of the clinical–anamnestic analysis showed that the shortest–lived and exacerbated heart failure as a cause of death was observed in the first group of children and the relatively long–lived in the 3rd group.For histological examination, fragments from different areas of both lungs were obtained during pathological anatomy. The macroscopic appearance of the lung was assessed by examining its appearance during autopsy. The lung fragments were hardened in 10% neutralized formalin, passed through alcohols, and paraffin was infused. Histological incisions were stained with hematoxylin–eosin, periodic acid Schiff reaction, and van–Gizon methods. The following morphometric calculations were performed:– the percentage of the area of the alveoli where air enters relative to the total area of the lung;– count the alveoli that have a glandular membrane in a field of view;– measurement of hyaline membrane thickness;– measurement of alveolar epithelial height;– measure the thickness of the interstitial alveolar tissue. Each indicator was calculated 10 times, and the mean index and the mean square error were calculated. Statistical processing of quantitative indicators was carried out by descriptive and variational statistical methods, the difference between the indicators was determined at the level of reliability R≤0.05.
3. Results and Discussion
The results of the study showed that the condition of infants in control group 1 was very severe at birth, with a profound lack of morphofunctional status, and severe heart and respiratory failure. The macroscopic condition of the lungs was determined to correspond to the gestational period. Histological examination revealed that in most cases, the epithelium of the alveolar tissue of this group of infants was large and their nucleus was normochrome stained in a round shape. However, in some cases, alveolar epithelium was damaged and the nucleus was deformed. In some areas of the lung, a small number of alveoli were found to be enlarged due to air intake, to form a round shape, as a result of which the alveocyte epithelium was flattened and elongated in shape. Other alveoli are almost unopened, in the form of various holes and cracks, the wall of their interstitium is thick and has a multicellular structure due to the accumulation of tissue–cells from the air (Fig. 1). The capillaries in the alveolar interstitial tissue are slightly dilated, filled with blood, and in some places there is a hemorrhage without diapedes (Fig. 2).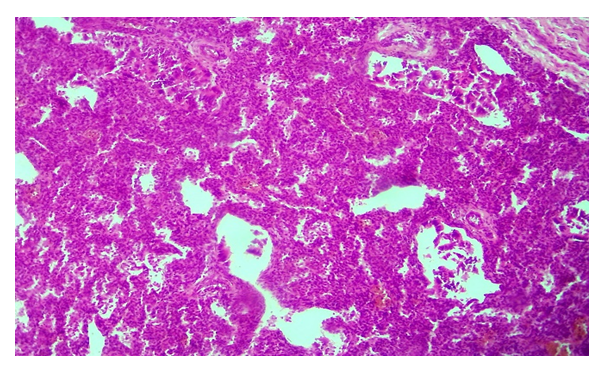 | Figure 1. A baby born at 25 weeks. Most alveoli are unopened. Snapshot: G-E. Scale: 10x10 |
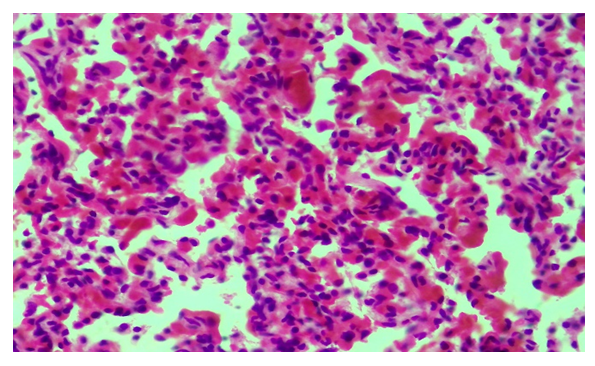 | Figure 2. Fragment of Figure 1. The capillaries are full, there is bleeding. Snapshot: G-E. Scale: 10х40 |
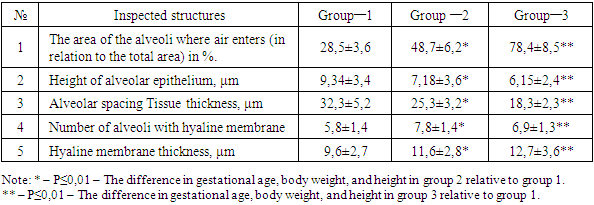
Morphometric measurements showed that the area of air–induced alveoli in group 1 was 28.5 ± 3.6% of the total area. The average number of alveoli with hyaline membranes in the cavity in a single field of view of the microscope was 5.8 ± 1.4. The height of alveocytes averaged 9.34 ± 3.4 μm, with alveocyte sizes ranging from 2.4 to 14.8 μm. In this group, the alveolar interstitial barrier thickness averaged 32.3 ± 5.2 μm, of which the thinnest was 9.6 μm and the thickest was 54.2 μm. The presence of hyaline membranes was found in 22.8% of the lungs of the first group of infants. It was observed that hyaline membrane fibers of different thicknesses, in some places spread in a lattice form. Measurements of hyaline membrane thickness showed an average of 9.6 ± 2.7 μm (Table 2), with a thickness difference of 2.6 μm for the thinnest area and 16.8 μm for the thickest area. According to the results of clinical–anamnestic analysis conducted in group 1, the presence of intranatal asphyxia, tumors of the brain and membranes, hemorrhage in most infants were identified as factors leading to hyaline membrane disease.In the second group, 3 premature infants died within the first 6 hours after birth, 6 (33.3%) between 12 and 24 hours, 5 (27.7%) between 72 hours, and another 4 (22.2%) between 120 hours was found dead at the time. Hyaline membranes with a homogeneous structure are detected in some areas of the lungs of 3 infants who die within 6 hours after birth. It was observed that the alveoli with a hyaline membrane in the cavity are located mainly in the peribronchial area. It was found that the hyaline membranes have a ring–shaped structure and the alveoli are tightly adhered to the inner surface. In addition to the hyaline membranes, amniotic fluid fragments, meconium bodies, and maternal erythrocytes were found in the alveolar cavity. Infants who died between 12 and 24 hours after birth were found to have hyaline membranes in most areas of lung tissue and to fill a relatively small alveolar cavity. It is observed that most alveoli are dilated, with the presence of hyaline membranes in the form of dark purple sand–sand in their cavity. Non–wrinkled alveoli lack hyaline membranes. In the wall of the alveoli, the reaction to hyaline membranes is found to be underdeveloped.Lung alveoli of infants who die within 72 hours after birth are observed to be of different sizes and some of them have hyaline membranes. In this case, the shape of the alveoli is triangular, elongated, star–shaped, in their cavity, in addition to hyaline membranes, amniotic epithelium, fibrin–like mass and segmented nuclear leukocytes are detected. It is observed that distalectase foci appear around the blood vessels and bronchioles, the blood vessels dilate, and the interstitial tissue and alveolar cavity become serous.In infants who died within 120 hours after birth, most areas of lung alveolar tissue were filled with air and dilated, emphysematous foci appeared in some areas, and hyaline membranes in the alveolar cavity were ruptured and fragmented. In this group, a strong reaction in the interstitial tissue in response to hyaline membranes, i.e. lympho–histiocytic infiltration, is detected. In the peribronchial and perivascular areas, small foci of atelectasis and distelectasis are found.Morphometric measurements of the lungs of the second group of infants showed that the area of air–induced alveoli was 48.7 ± 6.2% of the total area. The average number of alveoli with hyaline membranes in the cavity of a microscope field of view was 7.8 ± 1.4. The alveocyte height averaged 7.18 ± 3.6 μm, with alveocyte size ranging from 3.4 to 12.8 μm (Table 2). In this group, the alveolar interstitial barrier thickness averaged 25.3 ± 3.2 μm, of which the thinnest was 6.6 μm and the thickest was 42.4 μm. The second group of infants was found to have hyaline membranes in 27.8% of their lungs. It was observed that hyaline membrane fibers of different thicknesses, in some places spread in a lattice form. Measurements of hyaline membrane thickness showed an average of 11.6 ± 2.8 μm, with a thickness difference of 3.6 μm for the thinnest area and 21.8 μm for the thickest area.Table 2. Morphometric indicators of hyaline membrane disease (М±m)
 |
| |
|
In the third group, it was found that 8 (36.3%) premature babies died 12–24 hours after birth, 6 (27.3%) died within 72 hours, and another 8 (36.3%) died within 120 hours. In infants who died between 12 and 24 hours after birth, hyaline membranes were found in most areas of the lung tissue and filled the relatively enlarged alveolar cavity (Fig. 3). In the cavity of most alveoli, the presence of migrating alveocytes, segmented nuclear leukocytes, cell fragments, bronchial epithelium and lymphocytes is found. The alveoli of the lungs of babies that die within 72 hours of birth are of different sizes, and some of them have hyaline membranes. The alveolar interstitial tissue is thickened, in which fibroblasts and lymphoid cells proliferate (Fig. 4). The bronchial cavity is enlarged, contains a mass of leukocytes, migratory epithelium and erythrocytes. In infants who died within 120 hours of birth, most areas of the alveolar tissue of the lungs were filled with air, dilated emphysematous foci appeared in some areas, and the hyaline membranes in the alveolar cavity were torn and fragmented. In this group, there is also a strong reaction in the interstitial tissue, that is, lymphohistiocytic infiltration, in response to the hyaline membranes.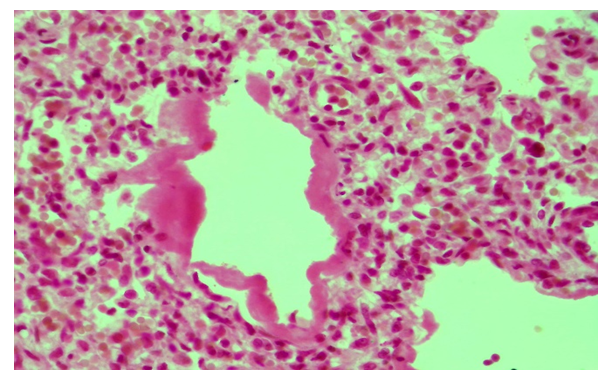 | Figure 3. A baby born at 34 weeks. Thick hyaline membrane in dilated alveoli. Snapshot: G-E. Scale: 10х40 |
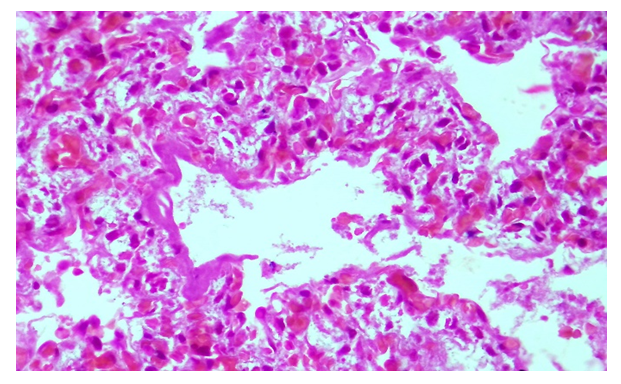 | Figure 4. A baby born at 36 weeks. The alveoli are enlarged, fibroblasts proliferate in the interstitial tissue. Snapshot: G–E. Scale: 10х40 |
The morphometric measurements performed on the lungs of the third group of infants showed that the area of air–induced alveoli was 78.4 ± 8.5 of the total area. The average number of alveoli with hyaline membranes in one cavity of the microscope was 6.9 ± 1.3. In this group, the alveolar interstitial barrier thickness averaged 18.3 ± 2.3 μm, of which the thinnest was 4.7 μm and the thickest was 38.4 μm. Measurements of the hyaline membrane thickness showed an average of 12.7 ± 3.6 μm, and the difference in thickness was found to be 5.6 μm for the thinnest area and 27.6 μm for the thickest area. The average height of alveocytes was 6.15 ± 2.4 μm, with alveocyte sizes ranging from 5.2 to 14.8 μm (Table 2).
4. Conclusions
Impairment of lung tissue, circulatory disorders, and damage to the alveolar and bronchial epithelium play an important role in the development of hyaline membrane disease in the lungs of premature infants. Accumulation of amniotic epithelium, meconium bodies, maternal erythrocytes in the alveolar cavity due to intranatal hypoxia and aspiration of amniotic fluid in premature infants due to damage to the alveolar epithelium is important. Morphometric examinations showed that only 1/3 of the lungs of infants born at 22-27 weeks had air in the alveoli, the alveolar interstitial tissue was thicker than in subsequent groups, the alveolar epithelium was relatively large, and the resulting hyaline membranes were relatively thin fibers. In the second group of infants who died at 28–32 weeks, the area occupied by the alveoli in the lungs doubled, the interstitial tissue thinned (25.3 ± 3.2), the alveocytes decreased, and the resulting hyaline membranes thickened relative to the previous group. In the third group of infants who died at 33–37 weeks, it was observed that the area of breathing alveoli in the lungs increased dramatically, accounting for 78.4 ± 8.5% of the total lung area. At the same time, it is found that the interstitial tissue is also thinner, the alveocytes are smaller, the hyaline membrane formed in the alveolar cavity is thicker than in other groups.
References
| [1] | Байбарина Е. Н., Антонов А. Г., Ленюшкина А. А. Клинические рекомендации по уходу за новорождёнными с экстремально низкой массой тела при рождении. Вопросы практической педиатрии 2006; 4 (1): 96–97. |
| [2] | Дементьева Г. М. Низкая масса тела при рождении. Гипоксия плода и новорождённого. Лекция для врачей. М.: МНИИ педиатрии и детской хирургии МЗ РФ; 2003. |
| [3] | Дементьева Г. М., Рюмина И. И., Фролова М. И. Выхаживание глубоко недоношенных детей: современное состояние проблемы. Педиатрия 2004; 3: 60–66. |
| [4] | Мостовой А. Профилактическое применение сурфактантов у новорождённых с экстремально низкой массой тела. Интенсивная терапия – Неонатология; 2:38, 2006. |
| [5] | Перетятко Л. П., Кулида Л. В., Проценко Е. В. Морфология плодов и новорождённых с экстремально низкой массой тела. Иваново; 2005. |
| [6] | Синельникова В. М., Антонов А. Г. Преждевременные роды. Недоношенный ребёнок, М.: ГЭОТАР_Медиа; 2006. |
| [7] | Greenough A., Milner A. D., Dimitrou G. Synchronized mechanical ven_tilation for respiratory support in newborn infants (Cochrane Review). The Cochrane Library 2002; 1: Update Software, Oxford. |
| [8] | Stevens T. P., Blennow M., Soll R. F. Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at riks for respiratory distress syndrome. The Cochrane Library, Copyright 2005, The Cochrane Collaboration. Volume 1; 2005. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
