-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(9): 948-952
doi:10.5923/j.ajmms.20221209.21
Received: Aug. 25, 2022; Accepted: Sep. 10, 2022; Published: Sep. 23, 2022

Predictors of Severe Diabetic Gastroparesis in Patients with 2 Type Diabetes Mellitus
Irodakhon Inoyatkhodjaeva1, Feruza Khaydarova1, Zufar Abdurakhmanov2
1Department of Diabetology, Republican Specialized Scientific and Practical Medical Center of Endocrinology, Tashkent, Uzbekistan
2Department of Surgical Diseases and Intensive Care, Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Zufar Abdurakhmanov, Department of Surgical Diseases and Intensive Care, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

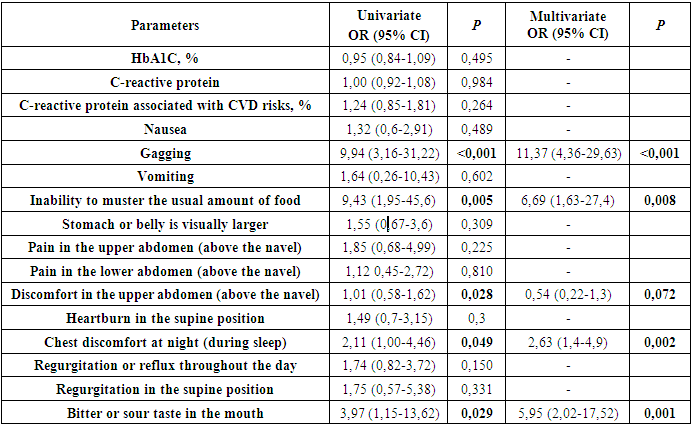
Diabetic gastroparesis is a heterogeneity of gastrointestinal symptoms ranging from asymptomatic manifestations up to mimicking by other nosological units which hampers to make a timely diagnosis. Objective. To identify the predictors of severe diabetic gastroparesis (DG) in patients with 2 type diabetes mellitus (DM2). Materials and methods. In the period from September 2020 to December 2021, 1026 patients with diabetes mellitus complicated by gastroparesis were treated. Taking into account the exclusion criteria (type 1 diabetes mellitus - 97 patients, thyroid-stimulating hormone level <0,1 and >4,5 μIU/ml - 23 patients, patients with mild DG according to the sum of the PAGI-SYM questionnaire scale ≤11-501 patients) 405 patients were included in this study. Data were collected including the definition of the clinical manifestation of gastropathy clarified by means of PAGI-SYM questionnaire. Univariate and multivariate analyzes were used to evaluate significant prognostic predictors of severe DG. Results. The mean age of the patients was 59,35±6,42 years with the duration of the underlying disease 12,39±4,84 years. The mean value of glycosylated hemoglobin was 10,12±1,76% [6,6–17,1%]. The average body mass index was 30,33±3,55 kg/m2. In multivariate analyse, vomiting (OR: 11,37; 95% CI: 4,36-29,63; p<0,001), the inability to master the usual portion of meal (OR: 6,69; 95% CI: 1,63-27,4; p<0,001), chest discomfort when lying down at night (OR: 2,63; 95% CI: 1,4-4,9; p<0,001), bitter or sour taste in the mouth (OR: 5,95; 95% CI: 2,02-17,52; p<0,001) were predictors of severe DG. It was also found that there is no correlation between the 5-, 10-, 15-year duration of the disease and severe degree of DG (p=0,833, p=0,623, p=0,553, respectively) which indicates the progression of gastroparesis regardless of the duration of DM2. Conclusions. Vomiting, the inability to master the usual portion of meal, chest discomfort when lying down at night, bitter or sour taste in the mouth are strongest predictors of severe DG regardless of the duration of DM2. Early diagnosis of DG with determining the severity of this complication can help to conduct timely conservative stage-related therapy in order to normalize the motor-evacuation function of the stomach.
Keywords: Diabetic gastroparesis, 2 type diabetes mellitus, PAGI-SYM questionnaire
Cite this paper: Irodakhon Inoyatkhodjaeva, Feruza Khaydarova, Zufar Abdurakhmanov, Predictors of Severe Diabetic Gastroparesis in Patients with 2 Type Diabetes Mellitus, American Journal of Medicine and Medical Sciences, Vol. 12 No. 9, 2022, pp. 948-952. doi: 10.5923/j.ajmms.20221209.21.
Article Outline
1. Introduction
- As known, DM2 poses a threat to the development of microangiopathy (neuropathy, nephropathy, retinopathy) and thus is considered as a consequential cause of deterioration in the life quality and reduction in life expectancy of patients [4,6]. Diabetic autonomic neuropathy is often not diagnosed and treated inadequately which itself can lead to cardiovascular autonomic neuropathy and gastrointestinal motility disorder - DG [8,12]. Unfortunately, clinicians pay insufficient attention to this chronic complication of DM2 because of its course under the "masks" of other nosological units that contributes to the difficulties of early diagnosis. The true prevalence of motor-evacuation dysfunction in patients with DM2 is probably underestimated. Kassander wrote in 1958 that "this syndrome is much more often 'missed' than diagnosed" [5]. To date, there are scarce data on the epidemiology of DG in patients with DM2, although this complication is not a rare occurrence and ranges from 25% to 70% of cases in the DM2 population [3,7,13].It has been suggested that the motor-evacuation dysfunction of the stomach does not affect the life expectancy of patients with DM2 [9]. Although DG in patients with DM2 is one of the reasons for the difficulty in achieving target glycaemic control and a decrease in the life quality of patients [10]. A slowdown in the evacuation of food from the stomach can contribute to postprandial hypoglycaemia followed by hyperglycaemia, which affects the development/progression of late complications of DM2 [12]. An increase in the frequency of hypoglycaemia in motor-evacuation dysfunction of the stomach in patients with DM2 is associated with a discrepancy between the peak action of prandial insulin and the rate of absorption of carbohydrates in the small intestine [1]. DG impairs also the bioavailability of oral medications due to the possible reflux of gastric contents into the respiratory tract that affects the treatment of comorbidities and increases the risk of complications during surgical interventions requiring anaesthesia [10]. Currently, there are no clear indications for instrumental research methods in order to screen disorders of the motor-evacuation function of the stomach in patients with DM2. In the process of collecting complaints and analysing the history of the disease (duration of DM2, dynamics of glycemia, lability of the course), it can be assumed that the patient has initial symptoms of DG. For a more accurate diagnosis of the asymptomatic course of this complication, Revicki D.A. and co-authors developed a specialized questionnaire which was tested in several multicentre studies and today has a high evidence of base [13]. Moreover, in the highly developed countries of the world, general practitioners and doctors of subspecialties use widely these questionnaires to assess the severity index of gastroparesis symptoms in patients with DM2 in daily medical practice. This diagnostic method also allows the patient to independently assess symptoms that have occur within two weeks of disease onset [2,14].The relationship between various gastrointestinal symptoms and the degree of motor-evacuation dysfunction, measures of glycaemic control which could serve as clinical markers or predictors of severe DG in patients with DM2 [11], is not well understood or is ambiguous.
2. Materials and Methods
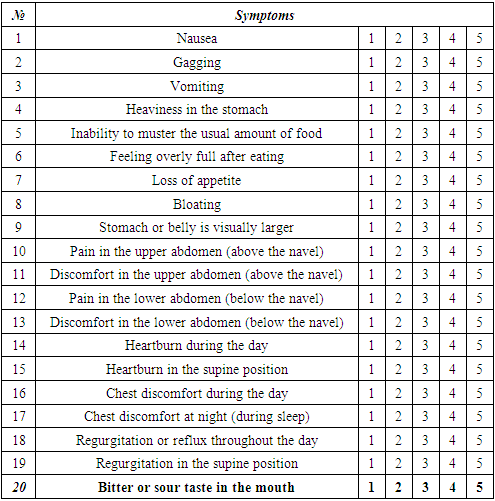
- The sample size was 1026 patients with diabetes mellitus complicated by gastroparesis. This prospective observational study included 405 patients with DM2 aged 18 to 75 years who were hospitalized at the Republican specialized scientific and practical medical center of endocrinology from September 2020 to December 2021. Exclusion criteria were type 1 diabetes mellitus - 97 patients, thyroid-stimulating hormone level <0,1 and >4,5 μIU/ml (23 patients), mild DG according to the sum of the PAGI-SYM questionnaire scale ≤11 (501 patients), whereas inclusion criteria - DM2, PAGI-SYM>20; thyroid-stimulating hormone 0,1-4,5 μIU/ml. The informed consent from patients was obtained. Data collection according to the questionnaire and scale measurement technique. The degree of DG was assessed using the PAGI-SYM questionnaire scale. The questionnaire consists of 20 questions, combining 6 subscales: heartburn/regurgitation (7 questions), nausea / vomiting (3 questions), feeling of postprandial fullness (postprandial distress syndrome)/early feeling of fullness (4 questions), meteorism/bloating (2 questions), pain in the upper abdomen (2 questions), pain in the lower abdomen (2 questions). The PAGI-SYM questionnaire allows the patient to assess subjective signs of symptom severity over a two-week period by assessing symptoms on a 5-point scale, where 0 means no symptoms, 1 is a minor symptom, 2 is a mild symptom, 3 is a severe symptom, 5 is more severe symptom. The sum of all points determines the degree of severity:mild degree (1–11 points);moderate degree (12–22 points);severe degree (23–33 points). Assessment of the symptoms of DG according to the PAGI-SYM questionnaire is presented in Table 1.
|
3. Results and Discussion
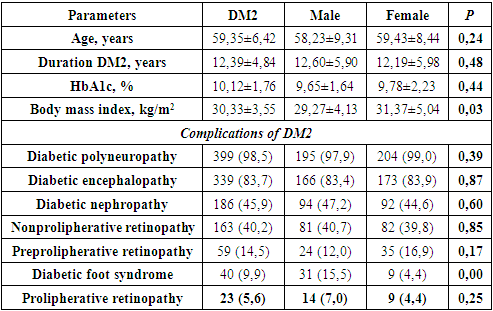
- To eliminate systematic errors, the subjects were stratified into two groups according to gender characteristics: the first group - 199 men; the second group - 206 women.The mean age of the patients was 59,35±6,42 years. Clinical characteristics of patients are shown in Table. 2.
|
3.1. Data According to Study of the PAGY-SYM Questionnaire
- The assessment of the scales of the PAGI-SYM questionnaire showed the presence of specific complaints including bloating, heaviness in the stomach, a feeling of fullness after eating, discomfort in the upper abdomen (above the navel), heartburn in a standing position, inability to master the usual amount of food, loss of appetite, bitter or sour taste in the mouth, discomfort in the chest during the day, discomfort in the lower abdomen (below the navel) in 93-99% of cases. According to the severity of symptoms, pain in the upper abdomen (above the navel), nausea, visually larger abdomen were identified in 80-87% of cases.All other least common symptoms of DG in 23-67% of cases were the following: pain in the lower abdomen (below the navel), food regurgitation when standing, discomfort in the chest at night, heartburn when lying down, feeling full of food/gagging (without vomit), food regurgitation in the supine position, vomiting. According to the PAGI-SYM questionnaire, the above symptoms of DG occurred equally, and the mean PAGI-SYM score did not differ between men and women.Moreover, the distribution of severe and moderate degree of DG among men and women with DM2 was identical (163 vs 180; p=0,215 and 36 vs 26; p=0,328, respectively).According to the results of the correlation analysis, gastrointestinal symptoms and C-reactive protein value had a direct correlation with severe DG, which were included in univariate and multivariate regression analyses. The identified predictors by means of regression analysis are presented in Table 3.
|
4. Conclusions
- Along with well-known complications such as diabetic polyneuropathy, retinopathy, nephropathy, DG is also the most common specific complication of DM2. Considering that DG can occur in any form (compensatory, sub-or decompensatory form) of DM2, clinicians should also pay attention and carry out timely complex treatment to eliminate this kind of complication, based on the identification of DG predictors (vomiting, inability to master the usual portion of food, discomfort in the chest in the supine position (at night), bitter or sour taste in the mouth).In conclusion, early diagnosis of DG, determining the severity of this complication, can help to conduct timely conservative stage-related therapy in order to normalize the motor-evacuation function of the stomach. Future studies should be focused on analysing the significance of the impact of conservative treatment of DG on glycaemic and lipid profile control and assessing the importance of including this therapy in the complex treatment of DM2.
ACKNOWLEDGEMENTS
- All authors have made significant contributions to the design, execution, analysis and writing of this study, and will share responsibility for the published material. Conflicts of interest: The authors declare no conflicts of interest.Ethics approval: This study was approved by the Institutional Review Board of both institutes, Uzbekistan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML