-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(9): 886-889
doi:10.5923/j.ajmms.20221209.06
Received: Aug. 13, 2022; Accepted: Aug. 26, 2022; Published: Sep. 15, 2022

Genetic Polymorphism in Position 197G/A (rs2275913) of IL 17A Gene in Patients with Diffuse Toxic Goiter
Kodirov A. E.1, Ziyadullaev Sh. Kh.1, Kim A. A.1, Kamalov Z. S.2, Ruzibakieva M. R.2, Olimzhonova F. J.3
1Samarkand State Medical University, Samarkand, Uzbekistan
2Institute of Immunology and Human Genomics of the Academy of Sciences of the Republic of Uzbekistan
3Tashkent State Dental Institute, Uzbekistan
Correspondence to: Kodirov A. E., Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diffuse toxic goiter is quite common in adults, relatively rare in childhood, but quite a serious disease. The leading role in the pathogenesis of DTG is given to the imbalance of the immune system, which leads to impaired immune control over the formation of autoantibodies, which underlies the autoimmune damage to thyroid cells. In the present study, the distribution of allele and genotype frequencies of the polymorphic region G-197A (rs2275913) of the IL-17A gene in patients with Graves' disease was studied and the contribution to the development of the disease was established. The population control group included 66 donors without thyroid pathology. Genotyping of the polymorphic region of the IL-17A immune response gene (rs2275913) was carried out by polymerase chain reaction (PCR) with allele-specific primers and electrophoretic detection of reaction products in agarose gel. From our results, the risk marker for the development of DTG is the A allele and the homozygous AA genotype. A significant increase in the A197 allele confirms the involvement of systemic inflammatory reactions in the pathogenesis of such a disease as diffuse toxic goiter.
Keywords: Toxic goiter, Thyroid gland, IL-17
Cite this paper: Kodirov A. E., Ziyadullaev Sh. Kh., Kim A. A., Kamalov Z. S., Ruzibakieva M. R., Olimzhonova F. J., Genetic Polymorphism in Position 197G/A (rs2275913) of IL 17A Gene in Patients with Diffuse Toxic Goiter, American Journal of Medicine and Medical Sciences, Vol. 12 No. 9, 2022, pp. 886-889. doi: 10.5923/j.ajmms.20221209.06.
1. Introduction
- Pathology of the thyroid gland (thyroid gland) is one of the most common diseases, outstripping even diabetes mellitus. The highest prevalence among thyroid diseases is due to diffuse toxic goiter (DTG), or Graves' disease [1,2]. The leading role in the pathogenesis of DTG is assigned to the imbalance of the immune system, which leads to violations of immune control over the formation of autoantibodies, which underlies the autoimmune lesion of thyroid cells [3,4]. The identification of genetic associations with DTG disease is justified, the interconnectedness, as well as the main significance of the MHC genes, the serine esterase gene of cytotoxic T lymphocytes, as well as the interleukin-1 receptor antagonist gene, the development of DTG. In the studied works, a negative interaction was revealed to identify the polymorphic marker His60Arg, a gene that encrypts the 3 subunit of the functional proteosome LMP2, as well as another polymorphic marker Pro52Thp, a gene that encrypts the thyrotropin interoceptor together with DTG [5,6]. In addition, the study of the relationship between the distribution of alleles, as well as genotypes in the group of healthy, as well as patients with DTG, polymorphic microsatellite marker, in the intron 2 antagonist of the IL-1 receptor, revealed differences in the frequency of occurrence of allele 2 and genotypes 2/4 and 4/4.One of the main roles in the study of genetic predisposition to the development of thyroid AIZ is the presence of this pathology in the family history [7,8]. Genomic screening studies have revealed candidate genes of predisposition to AIZ of 3 types: thyroid-specific genes, HLA complex genes, non-HLA immunoregulatory genes. The CTLA4 and PTPN22 genes belong to the latter group [9,10]. The CTLA 4 allele encodes a transmembrane regulatory protein that is expressed on activated T cells. CTLA 4 together with CD28 together with its ligand B7 on APC affects the activation of T cells, and also appears to be an important link for the stable interaction of T cells and APC. A number of studies on the Caucasian and Asian populations have shown the relationship of single nucleotide polymorphisms (SNP) A49G of the CTLA4 gene with the production of antibodies to the components of the thyroid tissue, susceptibility to DTG, as well as AIT [6,11].The interleukin-17 (IL-17) gene was first found in the cDNA of mouse T cells in 1993. The protein was initially called cytotoxic T-lymphocyte-associated antigens 8 (CTLA-8), because its amino acid sequence was very different from other cytokines known at that time, but had a high homology (57%) with an open reading frame of the Herpes virus saimiri T-lymphotropic virus [12]. In 1995, due to the discovery of a specific receptor for this protein, it was assigned to the class of interleukins [13]. A year later, it was shown that in rheumatoid arthritis, IL-17 induces the synthesis of other cytokines (IL-6 and IL-8) in synoviocytes [14]. These studies were the beginning of the study of the role of IL-17 in the development of immuno-inflammatory diseases. IL-17 plays a key role in protecting the body from extracellular bacterial and fungal infections [15]. However, excessive production of this protein is associated with immuno-inflammatory and autoimmune diseases (for example, psoriasis, psoriatic arthritis, rheumatoid arthritis, Bekhterev-Marie–Strümpell disease, systemic lupus erythematosus) [16].In connection with the above, the purpose of this study was to study the frequency distribution of alleles and genotypes of the polymorphic region G-197A (rs2275913) of the IL-17A gene in patients with Graves' disease and to determine the contribution to the development of the disease.
2. Materials and Methods
- The main group consisted of 97 patients with Graves' disease. The group of examined persons consisted of persons observed at the Department of Endocrinology of the Samarkand State Medical University. The studied control group included 66 donors without thyroid pathology.Venous blood from the ulnar vein served as the material for DNA isolation (Beckton-Dickinson vacutainers were used for blood sampling) with anticoagulant/preservative 15% tricalium EDTA (Ethylenediaminetetraacetic acid). Blood for further processing could be stored for up to 24 hours at a temperature no higher than +4°C. To obtain genomic DNA, a two-stage method of lysis of blood cells was used. Further purification of leukocyte mass lysates is based on the method of alcohol-salt treatment according to S. Miller et al. ((1988) in a modification proposed by the Stanford University Laboratory.Genotyping of polymorphic sections of immune response genes was carried out by polymerase chain reaction (PCR) with allele-specific primers 5'- ttcccattttccttcagaag [A/G] agagattcttctatgacctc - 3' (Scientific and Production Company “Litech”, Moscow) and electrophoretic detection of reaction products in agarose gel. We are tested SNP of IL-17 G197A (rs2275913). The identification of amplification products and their distribution with respect to the length marker was carried out in ultraviolet light (310 nm) after electrophoresis for 15 minutes at a voltage of 300 V (in both cases, the mileage was 3-4 cm) and staining with ethidium bromide.
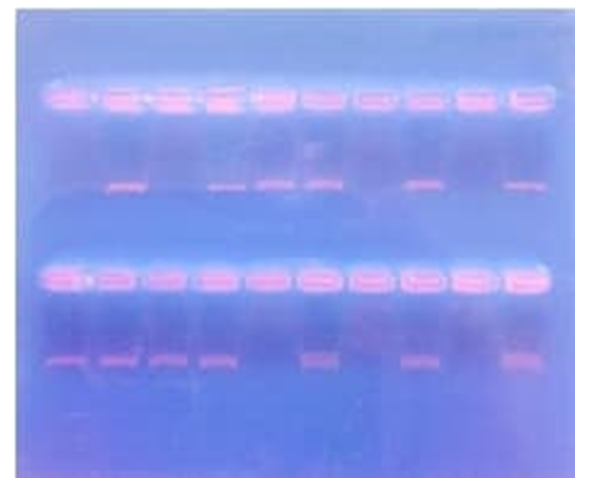 | Figure 1. An example of an electropherogram |
3. Results and Discussion
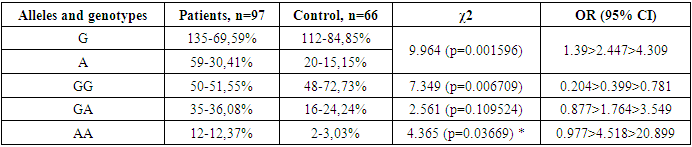
- In this study, we studied the nature of the frequency distribution of alleles and genotypes of the polymorphic variant G-197A (rs2275913) of the IL-17Au gene in patients with DTG. As can be seen from our results, the risk marker for the development of DTG is allele A and homozygous genotype AA (12.37% and 3.03%, respectively; OR = 4.518; 95% CI: 0.977 >4.518> 20.899; χ2=4.365 (p=0.03669)).
|
4. Conclusions
- Thus, a significant increase in the A 197 allele confirms the involvement of systemic inflammatory reactions in the pathogenesis of such a disease as diffuse toxic goiter.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML