-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(8): 848-853
doi:10.5923/j.ajmms.20221208.18
Received: Aug. 8, 2022; Accepted: Aug. 22, 2022; Published: Aug. 30, 2022

Influence of Various Variants of the Action of Furakumarins on the Exsudative and Proliferative Stages of Experimental Aseptic Inflammation
Abdukhalikova Nigora1, Iriskulov Bakhtiyor1, Sultanova Umida2, Saydalikhodjaeva Ozoda1
1Tashkent Medical Academy, Tashkent, Uzbekistan
2“Voice Lor Clinic” Private Clinic, Tashkent, Uzbekistan
Correspondence to: Abdukhalikova Nigora, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of this study was to study the effect of various schemes of using the photosensitizer-psoralen in the exudative and proliferative stages of inflammation, as well as the effect on morphological changes in local structures observed during inflammatory processes. Male rats were used in the experiment. It was shown that, under these conditions, the use of psoralen + UVR was superior to the use of psoralen itself. According to the morphological parameters, UVR in combination with psoralen showed the most effective anti-inflammatory results. The data obtained expand and deepen the understanding of the use of photosensitizers of plant origin in inflammatory processes in clinical conditions.
Keywords: Photodynamic therapy, Photosensitizers, Psoralen, Reactive oxygen species, Ultraviolet irradiation
Cite this paper: Abdukhalikova Nigora, Iriskulov Bakhtiyor, Sultanova Umida, Saydalikhodjaeva Ozoda, Influence of Various Variants of the Action of Furakumarins on the Exsudative and Proliferative Stages of Experimental Aseptic Inflammation, American Journal of Medicine and Medical Sciences, Vol. 12 No. 8, 2022, pp. 848-853. doi: 10.5923/j.ajmms.20221208.18.
Article Outline
1. Introduction
- Photodynamic therapy (PDT) is one of the promising areas of modern photobiology and has been undergoing rapid development in recent years due to new developments in the field of diagnosis, prevention and treatment of human diseases.The PDT method is based on the introduction into the body of chemicals - photosensitizers (PS), which have an increased tropism for target cells (cancer cells, inflammatory tissues, microbes and viruses) [1,2,3]. When exposed to light of a certain wavelength and energy, PS begin to produce atomic (singlet) oxygen, and also generates other reactive oxygen species (ROS), which cause oxidative damage to various molecules (proteins, unsaturated fatty acids, nucleic acids) and cellular structures (membranes), enzyme systems, genetic apparatus, etc.), which leads to the inactivation of pathogens [4]. Low doses of singlet oxygen, on the contrary, have a membrane-protective effect due to an increase in membrane permeability for Ca2+ with simultaneous activation of Ca2+-ATPase, which is responsible for the removal of Ca2+ from the cytoplasm and muscle relaxation [3]. It has been shown that this change in the activity of the enzyme, which improves the contractile function of the muscle, occurs precisely due to the accumulation of phospholipid hydroperoxides (primarily, the primary molecular products of lipid peroxidation) [5,6]. In addition, exposure to low doses of singlet oxygen can have an activating effect on cyclooxygenase, playing an important role in the kinetics of the enzymatic cascade of reactions that promote the formation of prostaglandins [7,8].Currently, PDT is actively used in many countries and has such advantages as low invasiveness, selective effect on the pathological focus, the possibility of repeating PDT courses, and low systemic toxicity [9,10,11]. The method has advantages over antimicrobial treatment, since the effectiveness of PDT is not related to the spectrum of sensitivity of microorganisms to antibiotics, it is successful even in the treatment of antibiotic-resistant microorganisms that do not develop resistance to PDT, in contrast to the effects of antibiotics. The bactericidal effect of PDT is local in nature, limited to the zone of laser irradiation of sensitized tissues, which makes it possible to avoid damage to the microflora characteristic of antibiotics in areas not exposed to irradiation, the possibility of repeated courses of treatment and combination in one diagnostic and therapeutic procedure [11].The method has found wide clinical application in oncology due to the selective accumulation of the photosensitizer in the tumor tissue. The drug is activated by local exposure to light with a wavelength corresponding to the maximum absorption, leading to the generation of singlet oxygen, which leads to damage to the intracellular structures of the tumor cell [12,13,14]. Currently, PDT has found wide application in many areas of medicine, the method is used to treat tumors of the skin, chest, lungs, bladder, infectious diseases, certain diseases of the skin and eyes, ENT organs, and oral cavity organs. The effectiveness of such treatment is high with minimal stress on the body [15].An additional advantage of PDT is its relative painlessness and the possibility of repetition on an outpatient basis.For photodynamic therapy, photosensitizers are used, which differ significantly in their physicochemical and photophysical properties, and the conditions for their photoactivation vary significantly [16,17].In recent years, despite the large number of synthetic drugs, medicinal plants occupy one of the first places in the production of medicinal products [18]. The value of natural biologically active substances (BAS) of plant origin is due to the fact that their chemical nature is close to the human body and is easily included in biochemical processes. As a rule, biologically active substances have a wide spectrum of biological activity and many of them have low toxicity.Psoralens are linear furocoumarins of plant or synthetic origin that sensitize biological objects to near ultraviolet radiation (UV-A radiation, 320–400 nm) [19]. The photosensitizing effect of psoralens is widely used in medicine in PUVA therapy (from the English P-psoralen and UV-A ultraviolet radiation of the A-spectrum) to treat diseases such as psoriasis, vitiligo, atopic dermatitis, eczema, cutaneous T-cell lymphoma, scleroderma (systemic sclerosis), graft-versus-host disease, and a number of other pathologies [20–25]. According to modern concepts, PUVA therapy is based on antiproliferative and proapoptotic effects on keratinocytes and immunocompetent cells, as well as the induction of immunosuppression, i.e., in essence, this method of treatment is photochemo- and photoimmunotherapeutic [20-25]. Literature sources report antibacterial, antidepressant, antioxidant, antiviral, antitumor, antimicrobial, anti-inflammatory and antipyretic effects, and psoralen is a calcium antagonist, affects the cell cycle, apoptosis, has estrogen-like, anti-osteoporotic effects [26,27,28].Psoralen is a toxic substance, especially for the liver. It can damage the liver through the formation of reactive furanoepoxide, which leads to irreversible inhibition of cytochrome P450, the main enzymatic system for the biotransformation of xenobiotics in hepatocytes [29]. With the combined use of psoralen with UV radiation, singlet oxygen is formed, which has an anti-inflammatory effect [3].Pure phospholipids, which form the structural basis of all cellular and intracellular membranes, do not absorb light in the visible and infrared regions of the spectrum characteristic of conventional laser radiation. But if a little sensitizer, psoralen, is added to phospholipids, then under the influence of light, for example, UV radiation, lipids are oxidized (photodynamic effect), accompanied by the formation of peroxides (photoperoxidation). LPO activation is a general universal reaction of cells to extreme impacts, a link in the pathogenesis of stress of any etiology [30]. However, the specificity of the inflammatory reaction consists, in particular, in the participation of specific cellular systems that destroy the cause of the inflammatory reaction (microorganisms, foreign bodies, own dead cells, etc.). These specific cellular systems include the system of phagocyte cells: neutrophils (polymorphonuclear leukocytes), mononuclear cells, macrophages, blood and tissue monocytes. These cells react to the appearance of microorganisms, dead and atypical cells with a "respiratory explosion" - a sharp increase in oxygen consumption and metabolic rate. As a result of a respiratory explosion, the phagocyte cell produces, using specialized enzyme systems, of which the main one is myeloperoxidase, significant amounts of reactive oxygen species: free radicals O2 * and *OH, hydrogen peroxide H2O2, and also ClO–, used by the cell to destroy microorganisms, cells -targets. The process ends with phagocytosis, intracellular digestion using lysosomal enzymes [31].
2. Purpose of the Research
- The purpose of this study is to study the effect of various regimens of psoralen use (with and without ultraviolet irradiation) on the exudative and proliferative stages of inflammation, and on morphological changes in local structures observed during inflammatory processes.
3. Materials and Methods
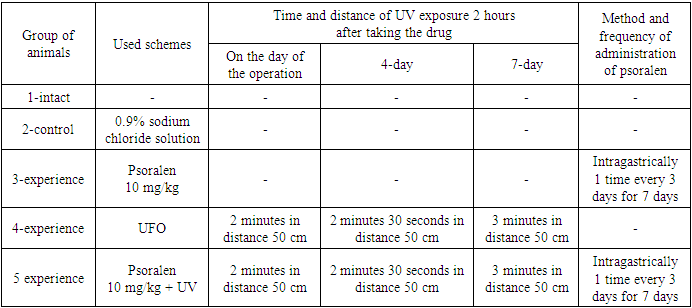
- In the experiment, male rats with an initial weight of 115-180 g were used, which were kept under standard vivarium conditions, quarantined for at least 12-14 days. The study of anti-inflammatory activity was carried out using the method of "felt granuloma". For the study, 5 groups of clinically healthy rats with clean skin were formed.Group 1: intact animals.Group 2: the control group of animals with a model of chronic proliferative inflammation, and received physiological saline intragastrically through a tube.Group 3: Animals with a chronic proliferative inflammation model treated with psoralen at a dose of 10 mg/kg.Group 4: Animals with a chronic proliferative inflammation model treated with ultraviolet irradiation with the same lamp, but without the use of psoralen.Group 5: Animals with a model of chronic proliferative inflammation, which were injected with psoralen at a dose of 10 mg/kg intragastrically through a tube and exposed to ultraviolet rays (UVR).1 day before the experiment, hair was carefully trimmed in the back area. The next day, under aseptic conditions under urethane anesthesia, a 1 cm long incision was made in the skin and subcutaneous tissue. Then, a cavity was formed through the formed skin incision in the subcutaneous tissue, where a pre-sterilized cotton ball weighing 10 mg was placed, after which 1 suture was applied.
|
4. Results and Discussion
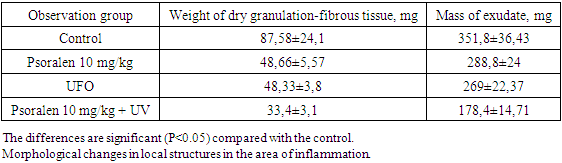
- Evaluation of anti-inflammatory activity showed that the development of granulomas was inhibited by all the studied procedures. When using psoralen 10 mg/kg without irradiation, the weight of dry granulation fibrous tissue was 48.66±5.57 mg, which was 44.2% lower than the control values. Thus, the mass of exudate was 288.8±24 mg, which is 17.6% less than that of the control group of animals (table 2). This means that psoralen 10 mg/kg+UVR was superior to psoralen 10 mg/kg without irradiation. So, when using a combination of psoralen and UVR, the mass of dry granulation-fibrous tissue was 33.4±3.1 mg, and the mass of exudate was 178.4±14.71 mg, which is lower than the control values by 61.5% and 49, respectively. 2%. And when using UV irradiation itself, the mass of dry granulation-fibrous tissue was 48.33±3.8 mg, and the mass of exudate was 269±22.37 mg. This is less than the control group by 44.5% and 23.3%, respectively (Table 2).
|
5. Conclusions
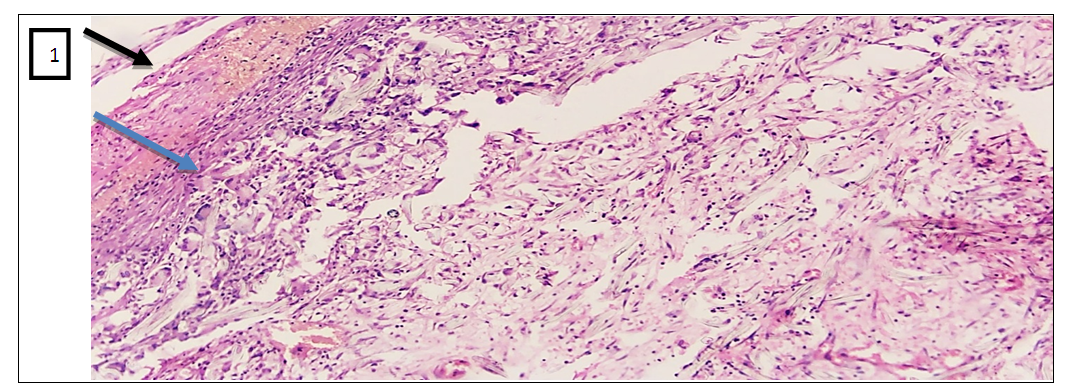
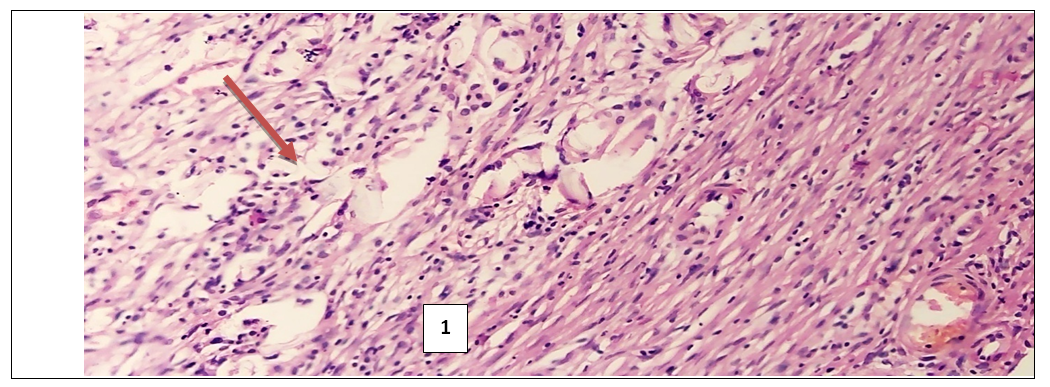
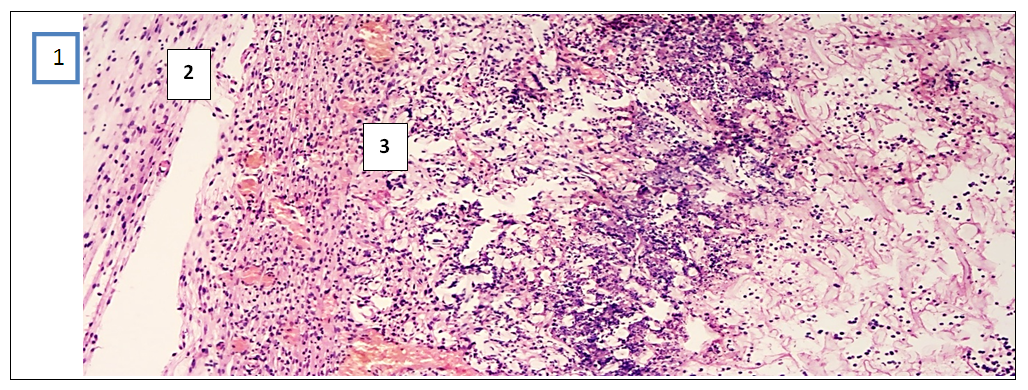
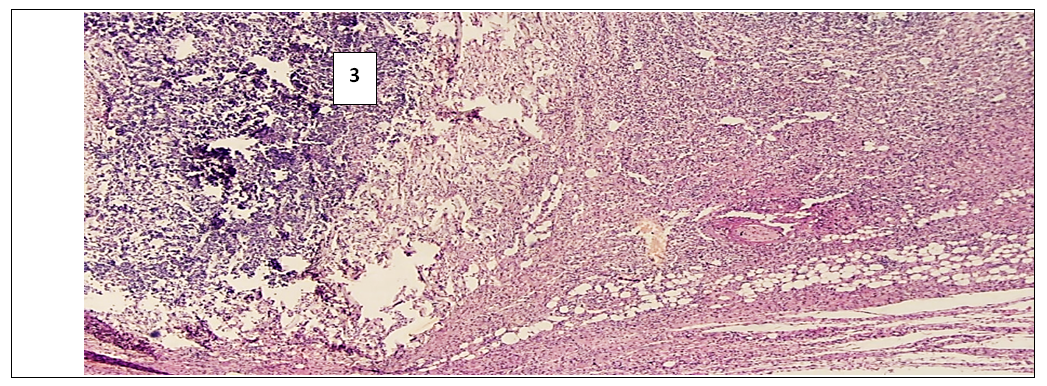
- In the experimental treatment of aseptic inflammation on the “felt granuloma” model, the results of the effectiveness of the proliferative and exudative activity of the studied tissues were significantly higher with the combination of psoralen and UVR.According to the morphological parameters, UVR in combination with psoralen showed the most effective anti-inflammatory results. This can be explained based on the following facts. First, in the sections in the group that used UVR in combination with psoralen, a clear fibrous capsule is noted, which is barely noticeable in the group that injected only psoralen. This means that the source of inflammation is isolated and the spread of the process to surrounding tissues is prevented. And in the UVR-only group, the fibrous cap is thicker compared to the other groups. But in the peripheral part of the capsule there are lymphocytic and xanthoma cells with diffuse spread to the nearby muscle tissue. While in the control group, which without treatment, there is a fibrous capsule with a transition to the zone of infiltration, angiomatosis and lysis of the soft tissue matrix, where xanthogranulomatous inflammation is located. This indicates the development of perifocal inflammation in the surrounding tissues of the inflammatory focus.Secondly, when using psoralen with UVR, there are multiple small blood vessels, when they are few in the application of psoralen without UVR. This means that the focus of inflammation is better supplied with blood and the flow of immune cells through the bloodstream increases. All this leads to the successful healing of the inflamed area. I would like to note the absence of the vascular layer in the control group for comparison.Thirdly, the ratio of the layer (1- fibrous capsule, 2-vascular layer, 3- inflammatory infiltrate) in the area of granulation inflammation in the combined use of psoralen and UVR is 1:1:1. And when using psoralen without UVR, the ratio of the fibrous wall to the inflammatory infiltrate is 0.5:2.5. This indicates that the infiltrate occupies more space in the focus of inflammation. When using UVR, this ratio is 1.5:1.5, and in the control group it is 1:0:2. This means the absence of the vascular layer, and indicates that the infiltrate takes up 1.5 and 2 times more space than other layers.Fourthly, the lowest content of xanthoma cells is noted in the sections of the group where the combination of psoralen and UVR was used. When the largest number of xanthoma cells in the area of granulation is noted in sections of the control group. Since xanthoma cells are macrophages that digest the decayed tissues captured by them, an increase in their number in the focus of inflammation means an acceleration of the degradation process in the focus of inflammation or provoking inflammation and accumulation of connective tissue.In inflammatory diseases, pathologically altered tissues never return to their original state as a result of proliferation. But in terms of morphological parameters, the combined use of psoralen and UVR in various treatment regimens conducted in our scientific study was closest to the parameters of healthy tissues. All of the above allows us to conclude that the combined use of the photosensitizer psoralen with UV radiation is relatively high.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML