-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(7): 719-723
doi:10.5923/j.ajmms.20221207.07
Received: May 25, 2022; Accepted: Jun. 15, 2022; Published: Jul. 15, 2022

Comparative Evaluation of Cytogenetic Changes in Bone Marrow Cells Under Chronic and Acute Irradiation in an Experiment
Tuxtayeva Khafiza Hikmatovna
Bukhara State Medical Institute, Uzbekistan
Correspondence to: Tuxtayeva Khafiza Hikmatovna, Bukhara State Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim was a comparative assessment of cytogenetic changes in the bone marrow cells of white mongrel rats under chronic and acute irradiation in an experiment. It was found that out of 125 examined bone marrow cells of white mongrel rats of the main group, normal metaphase plates were found in 48.0% of cells, the prophase stage was observed in 8.80% of cells, polyploid cells were found in 2.40% of cases, and cells with premature chromosome condensation were observed in 40.80% of cells. The presence of cells with pulverized chromosomes indicates the pathology of mitosis. The low content of cells with premature condensation of chromosomes and the absence of cells with pulverization of chromosomes indicates minor changes in the mitotic division of bone marrow cells of laboratory animals of the comparison group.
Keywords: Acute and chronic irradiation, Cytogenetic analysis of bone marrow cells, Experimental studies
Cite this paper: Tuxtayeva Khafiza Hikmatovna, Comparative Evaluation of Cytogenetic Changes in Bone Marrow Cells Under Chronic and Acute Irradiation in an Experiment, American Journal of Medicine and Medical Sciences, Vol. 12 No. 7, 2022, pp. 719-723. doi: 10.5923/j.ajmms.20221207.07.
Article Outline
1. Introduction
- Depending on the radiation dose and its distribution throughout the human or animal body, the timing and causes of their death vary. The most common is the bone marrow form of acute radiation sickness, while depending on the type of mammals, death occurs on 7-30 days from the moment of irradiation, and the causes of death are most often hemorrhagic syndrome or infectious complications [1,3]. Ionizing types of radiation include electromagnetic oscillations with a small wavelength, X-rays and gamma radiation, streams of α- and β-particles (electrons), protons, positrons, neutrons and other charged particles, α-radiation and X-ray radiation have a high penetrating power, β-radiation has a lower penetrating power [5]. Radioactive substances can enter the body through intact skin, gastrointestinal tract, respiratory organs. After that, they are carried by the blood and lymph current to organs and tissues [2,9]. It is proved that the hematopoiesis system of the body is most susceptible to the effects of radiation, especially for bone marrow cells. Under the influence of radiation, bone marrow aplasia develops, inhibition of mitotic processes in the organs of hematopoiesis, total death of low-grade bone marrow cells. A decrease in hematopoiesis is accompanied by the occurrence of hemorrhagic syndrome [2,5,8].Chronic radiation sickness is a complex clinical syndrome that develops in the case of prolonged exposure to ionizing radiation in doses that exceed the permissible. Characteristic manifestations: duration and undulation of the course; the presence in clinical symptoms of both signs of damage to the body from the effects of radiation, and manifestations of restorative and adaptive reactions. Periods of development of chronic radiation sickness: the period of formation, or actually chronic radiation sickness; the recovery period; the period of the consequences of radiation sickness [4,7].
2. The Aim of the Study
- The aim of the study was to study and compare the cytogenetic changes in the bone marrow cells of white mongrel rats under chronic and acute irradiation in the experiment.
3. Materials and Methods of Research
- To carry out the planned studies, 30 white mongrel male rats weighing 150-180 grams were used, kept in standard vivarium conditions (room temperature 21-22°C, relative humidity 50-60%, light mode - 12 hours of darkness and light). The maintenance of laboratory animals, feeding and caring for them, selection of animals, cleaning and disinfection of the vivarium premises were carried out according to Nuraliev N.A. et al. [6]. All laboratory animals (white mongrel rats) were obtained from the same nursery and of the same age. Before the start of experimental studies, all laboratory animals were kept in quarantine for 21 days. When working with experimental animals, all ethical principles of working with laboratory animals and rules of biological safety were strictly observed [1,6].All laboratory animals were divided into the following groups: The main group - white mongrel rats (n=12), who received chronic radiation for 20 days at 0.2 Gray daily; The comparison group consisted of white mongrel rats (n=12) who received acute radiation once at a dose of 5 Gray; The control group consisted of intact white mongrel rats (n=6) who did not receive acute and chronic radiation.Acute and chronic irradiation of laboratory animals was carried out using the gamma therapeutic apparatus AGAT-P1 (produced in Estonia, 1991), the source of irradiation is Co-60.During cytogenetic studies, all work with growth media and preparations was carried out in sterile conditions using a laminar box. Buffers were prepared on bidistilled water, filtered through membrane filters (0.22 microns "Millipor", Germany) and autoclaved at 1.2 atm. 30 minutes. The glassware is pre-sterilized at 1600C for 120 minutes before use. Equipment, fixtures, dishes made of polymer materials were exposed to ultraviolet light for 30 minutes. For experimental studies, bone marrow was selected from the femur of white mongrel rats during the autopsy of the animal. Cytogenetic changes in rat bone marrow cells were studied using the direct method. The method included the following steps: bone marrow was washed out of the femur of white mongrel rats involved in the experiment of all three study groups with RPMI 1640 nutrient medium with 0.04% colchicine (which destroys the spindle of division and chromosomes do not diverge to the poles during mitosis, forming a polyploid organism) into a centrifuge tube and incubated 2-2.5 hours in a thermostat at 37°C; incubated with hypotonic solution for 40 minutes in thermostat at 37°C; after hypotonization, a fixative was added three times in the proportion of one part of glacial acetic acid and three parts of 96-1000 ethyl alcohol; the resulting precipitate was applied to a pre-cleaned degreased slide and stained with Giemsa dye; metaphase was searched under a Leica microscope (Germany) at 200 times magnification, metaphase plates were analyzed at 1000 times magnification, 15 to 25 cells with metaphase plates were analyzed in each sample.Statistical data processing was carried out on a personal computer with the calculation of the mean values of the samples, variance, correlation coefficient. The reliability of the parameters was assessed using Student's t-test. The significance level of the indicator of the reliability of differences was considered P<0.05. When organizing and conducting research, the principles of evidence-based medicine were observed.
4. Research Results and Their Discussion
- For the analysis, we used bone marrow cells of laboratory animals that received and did not receive different types of radiation (acute and chronic), in which elements of the mitotic apparatus were detected (Table).
|
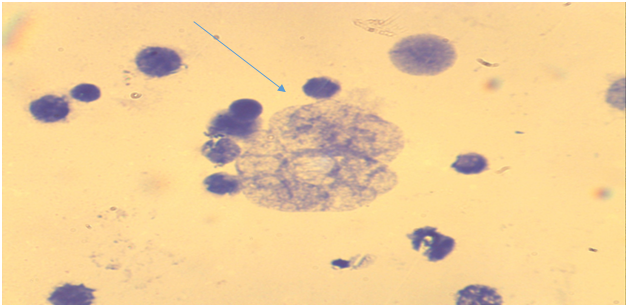
 | Figure 1. Bone marrow cells. Metaphase plate with normal karyotype (comparison group - acute irradiation, Approx. × 10, Vol. × 100) |
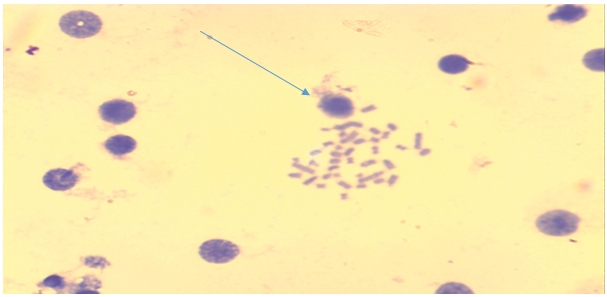 | Figure 2. Bone marrow cells. Normal early metaphase record (comparison group - acute irradiation, Approx. × 10, Vol. × 100) |
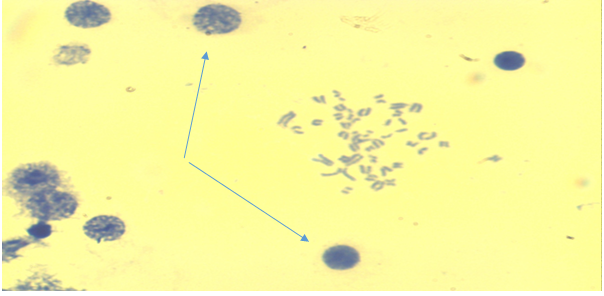
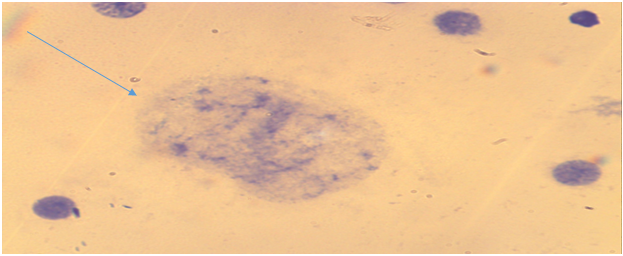 | Figure 3. Bone marrow cell. Premature condensation of chromosomes (the main group is chronic radiation exposure, Approx. × 10, Vol. × 100) |
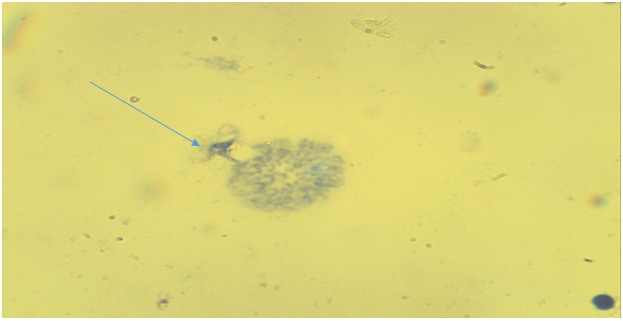 | Figure 4. Bone marrow cell. There is a late phase of premature chromosome condensation in the nucleus (the main group is chronic radiation exposure, Approx. × 10, Vol. × 100) |
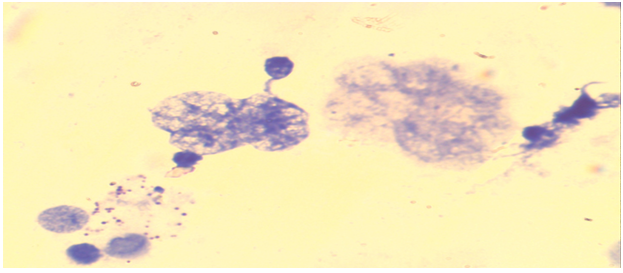
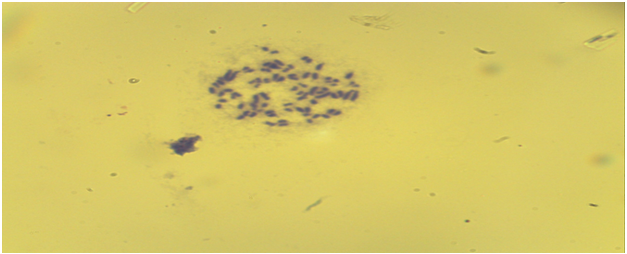 | Figure 7. Bone marrow cells. Normal karyotype, late metaphase ((control group - without acute and chronic irradiation, Approx. × 10, Vol. × 100) |
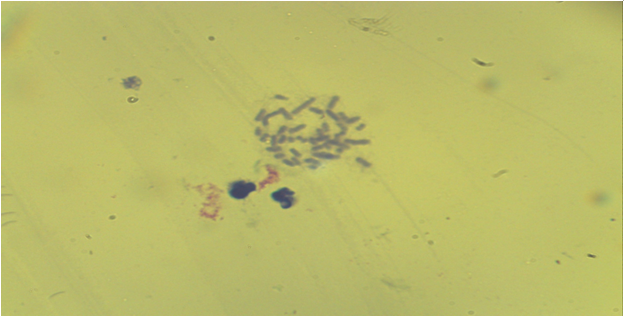 | Figure 8. Bone marrow cells. Normal karyotype, early metaphase ((control group - without acute and chronic irradiation, Approx. × 10, Vol. × 100) |
5. Conclusions
- 1. Out of 125 examined bone marrow cells of white mongrel rats of the main group (chronic irradiation), normal metaphase plates were found in 48.0% of cells, the prophase stage was observed in 8.80% of cells, polyploid cells were found in 2.40% of cases, cells with premature chromosome condensation were observed in 40.80% of cells. Of the 12 animals of the second group, 1 rat (8.33%) did not have mitotically dividing cells on the preparations, low cell count, low blast transformation and inhibition of mitosis were observed. The presence of cells with pulverized chromosomes indicates the pathology of mitosis.2. The presence of a high concentration of cells (40.80%) with premature condensation of chromosomes in the bone marrow cells of rats of the main group indicates the inhibition of the normal mitotic cycle, which affects the proliferative activity of this tissue and the presence of cell clones with genetic pathology.3. In the comparison group (acute irradiation), out of 123 examined bone marrow cells of laboratory animals, normal metaphase plates were detected in 72.36% of cells, 12.19% of cells were at the prophase stage. It should be emphasized that 5.69% of cells were polyploid (polyploidy), 9.76% of cells had premature condensation of chromosomes. 4. The low content of cells (9.76%) with premature condensation of chromosomes and the absence of cells with pulverization and scattering of chromosomes indicates minor changes in the mitotic division of bone marrow cells of laboratory animals of the comparison group. The absence of animals with low cellularity and low blast transformation (8.3%) indicates normal mitotic activity of bone marrow cells in all laboratory animals. This is due to the short observation period (5 days) of animals after acute irradiation.5. It is proved that after a single acute irradiation (at a dose of 5 Gray) during the first 5 days, there is practically no pathology of mitosis (changes in mitotic division of bone marrow cells), chromosomal aberrations do not appear, mitotic activity does not decrease. 6. In an experiment in laboratory animals after acute single irradiation (5 Gray), the severity of cytogenetic changes in bone marrow cells were less pronounced than with chronic irradiation. There were no deviations from normal processes in intact animals. Information about the source of support in the form of grants, equipment, and drugs. The authors did not receive financial support from manufacturers of medicines and medical equipment.Conflicts of interest: The authors have no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML