-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(6): 686-693
doi:10.5923/j.ajmms.20221206.16
Received: May 27, 2022; Accepted: Jun. 15, 2022; Published: Jun. 23, 2022

Proliferative Activity in vitro and Sample Preparation Evaluation in Conventional Cytogenetic Study in Patients with Hemoblastosis
Yuliana Yurevna Assesorova
Republican Specialized Scientific Practical Medical Center of Hematology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Republic of Uzbekistan
Correspondence to: Yuliana Yurevna Assesorova, Republican Specialized Scientific Practical Medical Center of Hematology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Republic of Uzbekistan.
| Email: | 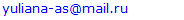 |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The success and effectiveness of conventional cytogenetic analysis (CСA) in patients with leukemia depends on the proliferative activity of leukemic cells in vitro and the related with it the number of metaphase plates on a microscopic preparation. The proliferative activity of leukemic cells in culture can vary significantly depending on the nosological form of leukemia, the individual characteristics of the course of the disease and the patient's condition. The aim of the study was to test a new assessment of the proliferative activity of cells and the number of metaphase plates in CСA. We have proposed a method for assessing cell growth based on counting metaphase plates on an area of a microscopic preparation equal to 287.5 mm2 (MFP/S287). The study revealed that this indicator is an objective criterion for the proliferative activity of cells cultured in vitro for CСA, and an effective tool for assessing the number of metaphase plates on a chromosomal preparation.
Keywords: Conventional cytogenetic analysis, Proliferative activity of leukemic cells, Metaphase plates
Cite this paper: Yuliana Yurevna Assesorova, Proliferative Activity in vitro and Sample Preparation Evaluation in Conventional Cytogenetic Study in Patients with Hemoblastosis, American Journal of Medicine and Medical Sciences, Vol. 12 No. 6, 2022, pp. 686-693. doi: 10.5923/j.ajmms.20221206.16.
1. Introduction
- One of the mandatory stages of examination of patients with leukemia is the conventional cytogenetic study (CCS), since the analysis of metaphase chromosomes using light microscopy allows us to assess the state of the entire karyotype and identify not only marker and recurrent, but also variant and additional chromosomal changes. The data of standard karyotyping make it possible to verify the diagnosis, stratify patients into risk groups and predict the course of the disease [1,2]. The analysis of the karyotype of leukemic cells is carried out after performing a number of technical tasks, including the cell cultivation in a nutrient medium to obtain the maximum possible number of cells at the metaphase stage, multi-stage isolation of metaphase plates from biological material, preparation and staining of cytogenetical glass slide; scanning of cytogenetical preparations using light microscopy, search and photo-preservation of metaphase plates suitable for cytogenetic analysis. The cytogenetic analysis itself consists in identifying all chromosomes within each metaphase plate under study in accordance with the International System of Cytogenomic Nomenclature (ISCN) [3], assessing their quantitative integrity and structural immutability, detection of chromosomal anomalies (aneuploidies and derivatives) and identifying of rearrangements. Nevertheless, in some cases, it is impossible to perform a CCS because of an insufficient number of metaphase plates. This fact may be due to both the unsatisfactory number of capable to division atypical mononuclears in the biomaterial and the extremely low proliferative activity of leukemia cells.However, even the high proliferative activity of tumor cells does not guarantee obtaining a sufficient number of metaphase plates suitable for karyotype analysis. The presence of a cell in one or another stage of division depends on the duration of the cell cycle, which varies significantly among patients with hematological neoplasia. So, there is evidence that among patients with acute myeloid leukemia, the range of one cycle can be from 16 hours to 292 hours [4]. The duration of the cell cycle of individual tumor clones is unknown, therefore, cell division can be forcibly stopped using colchicine or its analogues not only when the chromosomes are in a state of maximum chromatin condensation and are located in the equatorial plane of the cell, but also in early metaphase. As a result, cytogenetic preparations, in addition to individual metaphase plates scattered among interphase nuclei, will also contain clusters of weakly spiralized and morphologically undifferentiated chromosomes.The experience of karyotype studies in leukemia patients conducted by us with the use of CCS shows that despite the same conditions of cultivation in nutrient media and the standard method of preparation of chromosomal specimens, the number of metaphase plates has significant differences both in patients with different types of hemoblastosis and in patients with the same nosological form of the disease. The data of the scientific literature adduce various reasons for the variability of proliferative activity of cells in vitro [5,6,7], however, we have not found a convincing explanation for this fact in relation to leukemic cells. It is probably necessary to conduct a series of studies to understand the mechanisms of loss of growth activity of these cells during cultivation in vitro. At the same time, the technique of objective assessment of cell growth and the associated number of metaphase plates should become a necessary tool for such studies. In this regard, the aim of this study was to develop a method that allows to objectively assess the number of metaphase plates obtained from blood and bone marrow cells cultured in vitro.
2. Methods
- The study was carried out on the basis of the Laboratory of Molecular Genetics and Cytogenetics of the Republican Specialized Scientific and Practical Medical Center of Hematology (RSSPMCH) of the Ministry of Health of the Republic of Uzbekistan in 2019-2021. The object of the study was metaphase plates obtained from leukemia cells of blood (BC) and bone marrow (BMC) of patients with chronic myeloproliferative neoplasia (CMPN) (n=150), as well as from mitogen-stimulated T-lymphocytes of blood of persons without hematological neoplasia (n=84).In vitro cultivation of BMC and BC of patients with CMPN was carried out in a nutrient medium containing 5 ml of RPMI-1640 with glutamine, 1 ml of fetal bovine serum (FBS), 6 ml of 0.1% colchicine solution; cultivation was carried out for 21 hours at a temperature of 37.0°C. In vitro cultivation of BC of subjects without hematological neoplasia was carried out in a nutrient medium containing 5 ml of RPMI-1640 with glutamine, 1 ml of FBS, 35 ml of 0.1% solution of the phytohemagglutinin (PHA) mitogen. Cell division was stopped at the metaphase stage with 0.1% colchicine solution, which was added to the medium in an amount of 40 ml 30 minutes before the beginning of hypotension. Cultivation was carried out for 72 hours at a temperature of 37.0°C.The cultured biomaterial was precipitated by centrifugation (1500 turn in minute, 7 min). A 0.56% solution of potassium chloride was added to the cellular sediment and incubated for 25 minutes at a temperature of 37.0°C. After the incubation was completed, the biomaterial was fixed three times using ethanol and glacial acetic acid in a ratio of 3:1 – to obtain a precipitate containing metaphase plates. Cytogenetic specimens were prepared by dripping drops of cooled suspended sediment onto a glass slide from a height of 50 cm; the specimens were dried at room temperature for 24 hours. Differential staining (GTG-binding) of cytogenetic preparations was performed using trypsin (0.025 g / 10 ml of distilled water) and Giemsa dye diluted on a phosphate buffer (PhB) (0.5 ml dye: 10 ml PhB). The proliferative activity of cultured in vitro cells was evaluated using a direct light microscope ZEISS Axio Scope. A1 with magnification of the microscope of ×200 by counting the number of metaphase plates (MFP) per unit surface area of the chromosomal glass slide (S) equal to 287.5 mm2.Statistical analysis of the data obtained was carried out using the calculation of statistical indicators of the standard deviation (σ), Student's t-test when comparing relative and average values. The differences were considered statistically significant at p<0.05.
3. Results
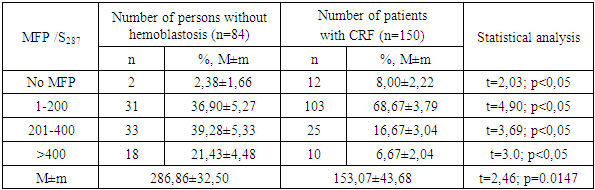
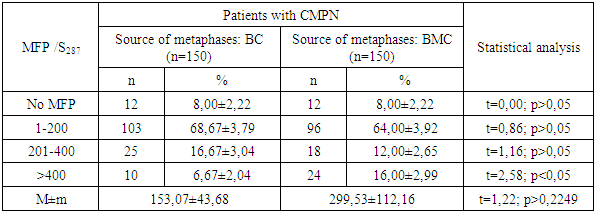
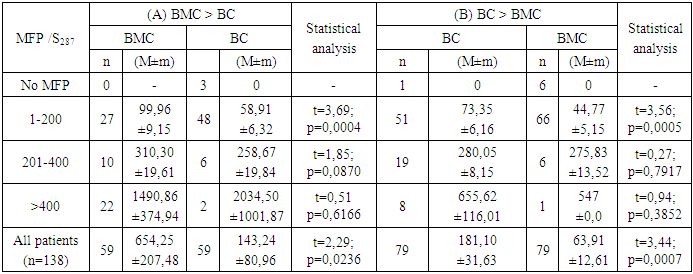
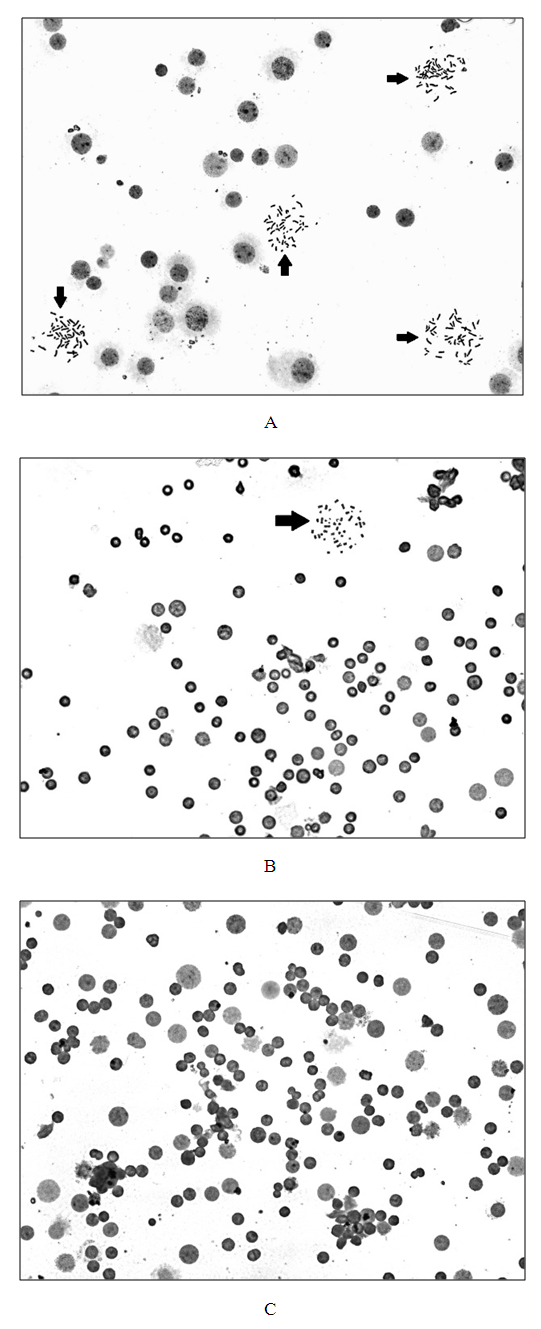
- The evaluation of the proliferative activity of in vitro cultured leukemia cells of patients with chronic myeloproliferative neoplasia (CMPN), as well as mitogen-stimulated lymphocytes of conditionally healthy individuals was carried out by counting the number of metaphase plates (MFP) on the surface area of the chromosomal glass slide (S), which is equal to the visual field of ten transverse scans of a standard slide with a width of 25 mm when magnified by a microscope ×200.The scanned surface area of the chromosomal preparation (S, mm2) was calculated according to the formula developed by us (1):
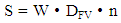 | (1) |
 | (2) |
|
|
|
4. Discussion
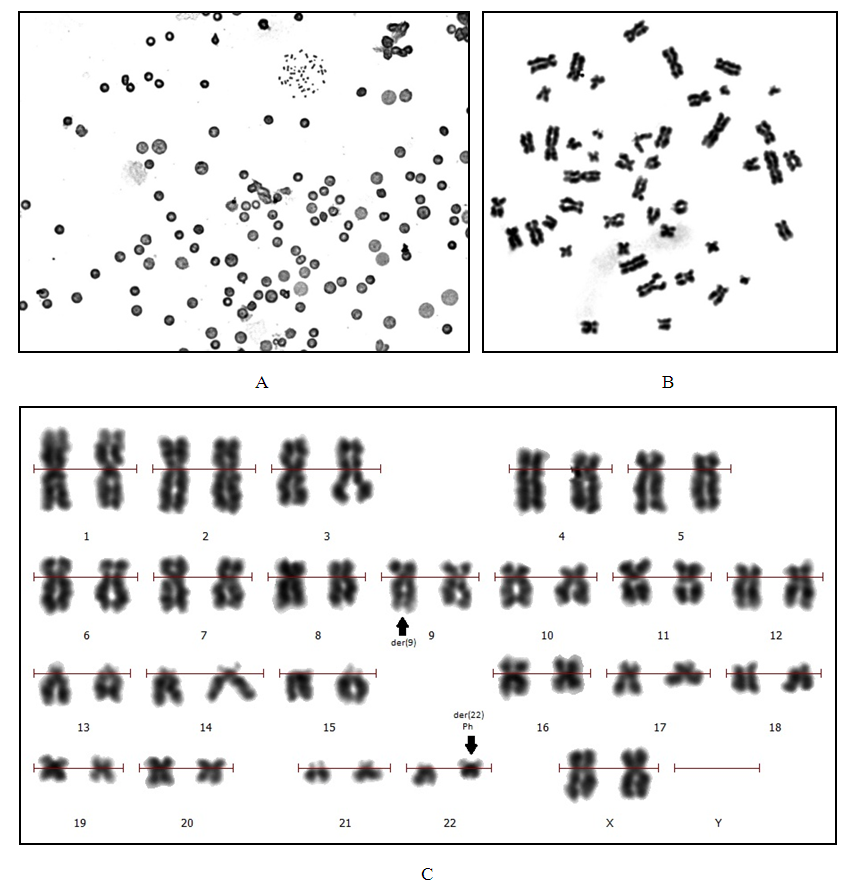
- The determination of karyotype abnormalities is an integral part of modern hematology: cytogenetic analysis data are among the mandatory differential diagnostic criteria [10,16]. Thus, the diagnosis of chronic myeloid leukemia (CML) is established on the basis of clinical and laboratory studies with the mandatory detection of t(9;22)(q34;q11.2), the cytogenetic sign of which are derivatives of chromosomes 9 and 22 (Ph-chromosome) (Figure 1) [17,18,19,20].
5. Conclusions
- Despite the fact that the ratio of high-tech molecular genetic methods in the study of pathogenetic mechanisms of neoplastic diseases of the hematopoiesis system is huge, the cytogenetic method based on differential chromosome staining has not lost its importance. Classical karyotyping, based on cellular technologies, allows obtaining valuable information about the state of the karyotype, and serves as a conditional link between the molecular and organismic levels of human organization. Having certain advantages, the conventional cytogenetic method has a number of limitations, overcoming which can significantly increase its effectiveness. The search for opportunities to control the proliferative activity of leukemia cells in culture conditions in order to obtain metaphase plates necessary for karyotype analysis is one of the ways to optimize this technology.
ACKNOWLEDGEMENTS
- The author of the paper expresses gratitude to the administration and staff of the Laboratory of Molecular Genetics and Cytogenetics for providing the opportunity to perform the study and its technical support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML