-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(6): 616-638
doi:10.5923/j.ajmms.20221206.05
Received: May 10, 2022; Accepted: May 29, 2022; Published: Jun. 13, 2022

Towards the Misuse of Advanced Wireless Sensor Technology to Enable the Sudden Onset of ARDS
Md Rahimullah Miah1, 2, Md Mehedi Hasan3, Jorin Tasnim Parisha4, Chowdhury Shadman Shahriar5, Alexander Kiew Sayok2, Shahriar Hussain Chowdhury6
1Department of IT in Health, North East Medical College and Hospital, Affiliated with Sylhet Medical University, (SMU), Sylhet, Bangladesh. and PhD Awardee from the IBEC, UNIMAS, Sarawak, Malaysia
2IBEC, Universiti Malaysia Sarawak (UNIMAS), Kota Samarahan, Sarawak, Malaysia
3Department of Law, Green University of Bangladesh, Dhaka, Bangladesh
4Government S.C. Girls’ High School, Sunamganj Sadar, Sunamganj, Bangladesh
5USMLE Student, USA and Ex-student of North East Medical College, Sylhet, Bangladesh (BD)
6Department of Dermatology, North East Medical College and Hospital, Affiliated with SMU, Sylhet, Bangladesh
Correspondence to: Md Rahimullah Miah, Department of IT in Health, North East Medical College and Hospital, Affiliated with Sylhet Medical University, (SMU), Sylhet, Bangladesh. and PhD Awardee from the IBEC, UNIMAS, Sarawak, Malaysia.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Patients with sudden acute respiratory distress syndrome (SARDS) are characterized by changes in pulmonary edema associated with endothelial and epithelial permeability. Sudden acute respiratory failure in ARDS patients is highly prevalent and potentially prognostic due to misuse of wireless sensor. The study was conducted among individuals with BMI categories through wireless sensor tracking due to active open eyes, self-voice and a specific GPS location. Everyone uses advanced wireless technology but no one can be aware of its security. Studies have illustrated the misuse of wireless sensor device with in-body GPS sensor, individual’s lungs affect suddenly at the fixed GPS coordinates. The excess weight patients attack on the sudden onset of ARDS than that of other categories of BMI. These results reflect the importance of protecting human health that the State provides. For SARDS management and disease-free living for all, everyone's wireless sensor technical knowledge was essential, but such knowledge was insufficient. The study is a very timely scientific research- it will benefit those around the world who take it. There is no doubt about this research, but cyber criminals can create suspicion among others through artificial intelligence. So, everyone should be aware of this research positively.
Keywords: Active wireless sensor, Active open eye, Self-voice, GPS location
Cite this paper: Md Rahimullah Miah, Md Mehedi Hasan, Jorin Tasnim Parisha, Chowdhury Shadman Shahriar, Alexander Kiew Sayok, Shahriar Hussain Chowdhury, Towards the Misuse of Advanced Wireless Sensor Technology to Enable the Sudden Onset of ARDS, American Journal of Medicine and Medical Sciences, Vol. 12 No. 6, 2022, pp. 616-638. doi: 10.5923/j.ajmms.20221206.05.
Article Outline
1. Introduction
- Breathing is essential for the survival of all human beings and animals on earth. We all move comfortably to certain GPS locations, balancing the body's oxygen and atmospheric oxygen. This GPS location is controlled by light, word of mouth, thinking and advanced technology. This is because the electromagnetic force in them works in a certain proportion and keeps each of us alive. But when this balance is lacking, each of us suffers from respiratory disease. ARDS is one type of respiratory diseases [1],[2], which can be determined through this study. Sudden Acute Respiratory Distress Syndrome (SARDS) refers to a state of sudden respiratory failure due to fluid accumulation in the lungs and acute inflammation [1,2,3,4,5,6,7,8,9,10]. SARDS is a serious life-threatening problem that causes the current death rate to be around 100 percent [11],[12],[13],[14],[15],[16]; [17],[18],[19],[20],[21]. This condition is also medically called sudden shock lung, because it occurs after an abnormal sudden condition that leads to a state of shock, such as traumatic injury [22-35]. Suddenly experiencing such a syndrome, SARDS is an unimaginable concept of an underlying medical condition, usually a fatal phenomenon that causes blood, fluid, and tissue to cross the barrier and allow air to enter the lung cells, causing them to rupture [36-75]. Once the alveoli are compromised in this way, breathing becomes difficult and ultimately impossible without rapid treatment [76-99]. Many people have shortness of breath due to cold and cough suddenly [100-110]. At present almost, all patients with coronavirus disease are heard to talk about shortness of breath [111-119]. Different reasons can cause shortness of breath. Mainly cold-cough, pneumonia, bronchitis, causes of heart disease, stomach problems, gas and digestive problems, allergies, asthma, anemia, excessive stress and even shortness of breath in tension [120-125]. However, in most cases, lung problems are responsible [126-129]. Some internal health problems can also cause shortness of breath [130-132]. There may also be temporary shortness of breath [133-135]. Occasionally there may be mild shortness of breath due to nasal congestion [136-140]. If so, it can be easily managed at home [141-145]. But if people have regular shortness of breath or a lot of problems, they must consult a doctor [146-150]. Sometimes, individuals suffer from sudden acute respiratory syndrome, but cannot recover it through doctor's advice or medication, meanwhile the patient was anxious and eventually died [151-155]. According to some doctors, environmental pollution is high in the city [156-160]. This disease of the lungs spreads rapidly due to environmental pollution like dust and washing [161-166]. People with asthma suffer more than others [167-170]. Asthma can be exacerbated in those who have fewer problems [171-175]. One of the other reasons for the increase in asthma in the city is overcrowding [176-179]. Many people in the city have to live in very crowded houses which makes the indoor environment damp and unhealthy [180-185]. In the corners of the house, the dust accumulated on the floor of the furniture and in that dust a kind of insect called 'dust mite' is made more [186-189]. Dust and these worms increase asthma [190-193]. The smoke from cooking also pollutes the indoor environment [194-196]. Closed rooms in the city do not have ventilation systems, especially for women and children [197-200]. These problems are lessened due to open environment in rural areas [201-202]. The changes that take place in people's lives in the city, such as the introduction of many artificial foods instead of fresh food [102]. Exercise and physical activity are also reduced in the city [108]. In the case of children in particular, pollution as well as lack of sports facilities, life in captivity hinders the vigorous growth of their lungs [203-205]. Weak lungs are more likely to cause asthma [206-210]. After hard work we continue to breathe [211-212]. Everyone's breathing is faster if they run or work hard [213-215]. But it is not shortness of breath. If there is an increase in the rate of breathing as well as difficulty in breathing and exhaling, then it is called shortness of breath [216-220]. This is actually a symptom of the disease [221-225]. Proper health service and treatment is much more likely to cure this problem [226-230].The study is to find out the evidence of misusing of advanced wireless sensor technology to recover the sudden onset ARDS with core challenges in public health security worldwide.
2. Materials and Methods
2.1. Study Tools
- The study followed the materials and methods from the URLs [182-196]:a. URL: http://article.sapub.org/10.5923.j.geo.20211101.02.html [195]b. URL: http://article.sapub.org/10.5923.j.ijvmb.20211001.03.html [190].c. URL: https://ir.unimas.my/id/eprint/24535/ [182]d. URL: http://article.sapub.org/10.5923.j.ajbe.20201001.03.html [192]e. URL: http://article.sapub.org/10.5923.j.bioinformatics.20211101.01.html [191]f. URL: http://article.sapub.org/10.5923.j.fs.20211101.01.html [194]g. URL: https://doi.org/10.30564/jer.v3i1.2826 [184]h. URL: http://article.sapub.org/10.5923.j.diabetes.20200902.02.html [188]i. URL: http://article.sapub.org/10.5923.j.ijas.20211102.02.html [186]j. URL: http://article.sapub.org/10.5923.j.scit.20211101.02.html [193]k. URL: http://article.sapub.org/10.5923.j.env.20211102.01.html [185]l. URL: https://ccsenet.org/journal/index.php/gjhs/article/view/0/46717 [196]m. URL: http://article.sapub.org/10.5923.j.ijim.20221101.01.html [187]n. URL: https://www.ccsenet.org/journal/index.php/jsd/article/view/0/40313 [189]Sensor Tracking towards lungs included different steps, which as shown in Figure 1.
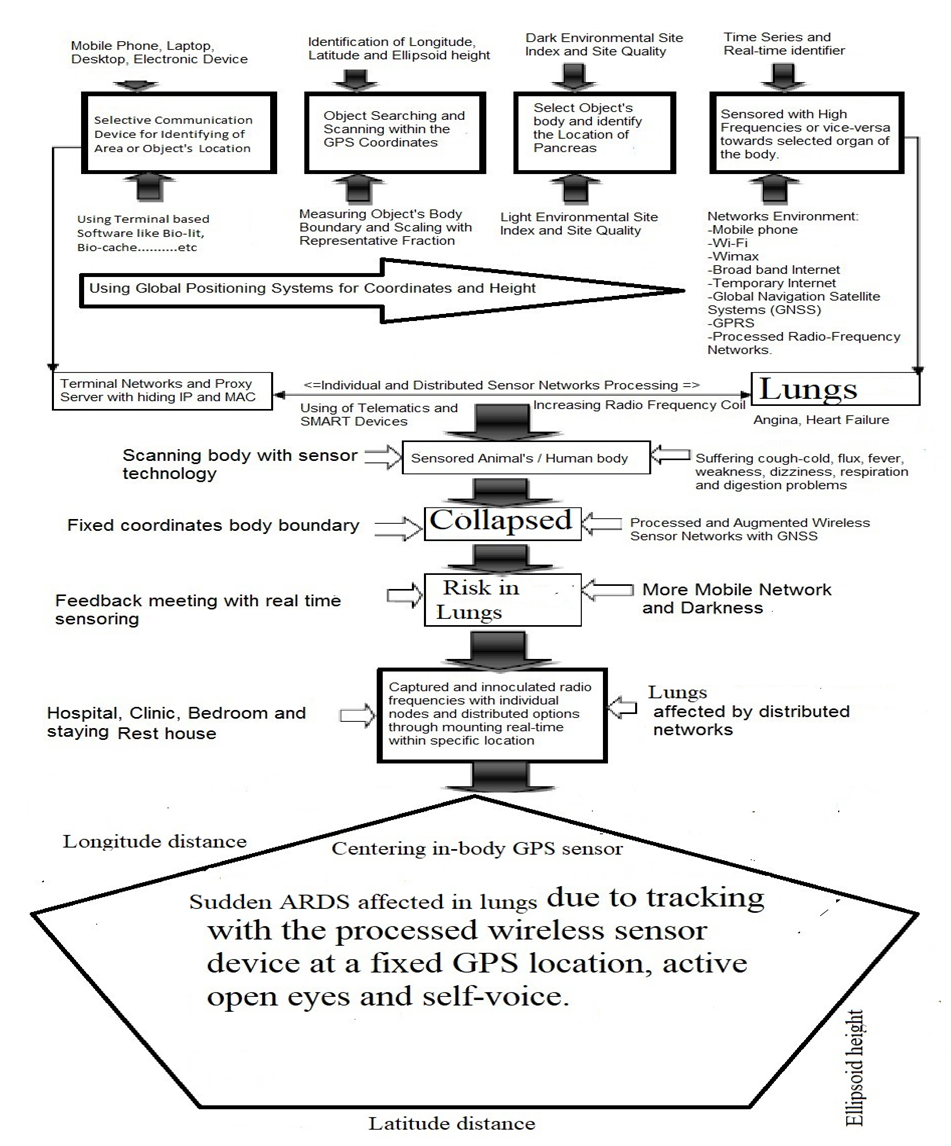 | Figure 1. Sensor Tracking towards lungs of selected animals |
2.2. Study Site
- The study site of this research was conducted at the Universiti Malaysia Sarawak (UNIMAS), Sarawak, Malaysia from October 8, 2014 to May 21, 2018 as a part of PhD degree. The study follows the different parameters on sample size and ISNAH (Impact of Sensor Networks towards Animals, Human beings) data size and design, tracking procedure, data compilation and analysis related to the Sudden ARDS towards cat and dog due to misuse the advanced wireless sensor technology worldwide, which as shown in Figure 2.
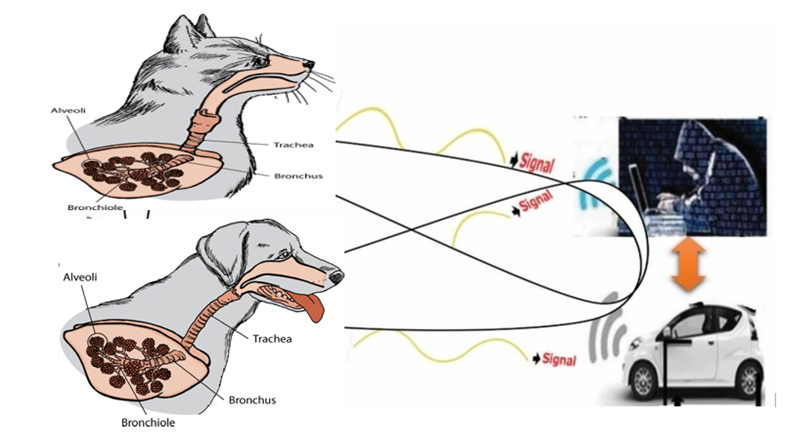 | Figure 2. Sudden onset ARDS with wireless sensor technology towards lungs of animals |
2.3. Data Size and Design
- The research presented in different parameters including 7 cats and 7 dogs individually with the design of ISNAH experiment. The study followed the tracking system towards animals to identify the effect of the processed wireless sensor networks towards them separately.
2.4. ISNAH Procedures
- Primary and secondary health data collection procedures are diverse. The study identified the impact of advanced wireless sensor technology on cardiac arrest with GPS locations and GNSS positions according to research objectives from ISNAH procedure. The steps of this procedure illustrated in Figure 3.
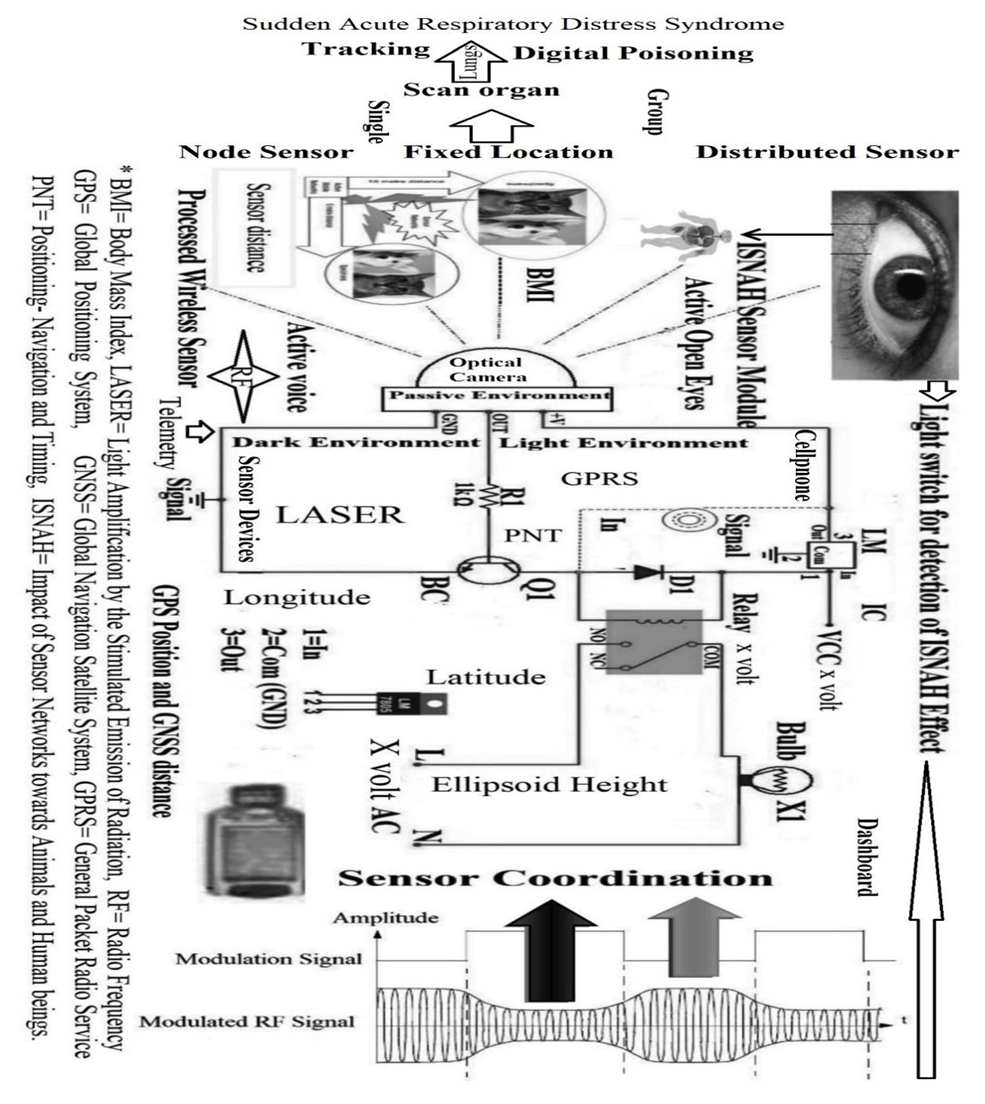 | Figure 3. The procedures of ISNAH |
2.5. Diverse Tracking Process
- The diverse tracking procedures include in different stages with ISNAH experiment from built-in sensor device, particularly identification of fixed GPS locations including longitude, latitude and ellipsoid height, which as shown in Figure 4 towards dogs and in Figure 5 towards cats. The wireless sensor tracking systems included at a fixed GPS location and GNSS distances of animals in required stages. The processed wireless sensor networks tracked animals for digital poisoning in different GPS and GNSS locations including (i) ellipsoid height, (ii) longitudinal distance, and (iii) adjacent latitude. The tracking parameter included (a) open active eyes cats and dogs, (b) tightly closed eyes of cats and dogs, (c) at dark environment, (d) at light environment, (e) selection on the category of FBMI (Feline Body Mass Index).
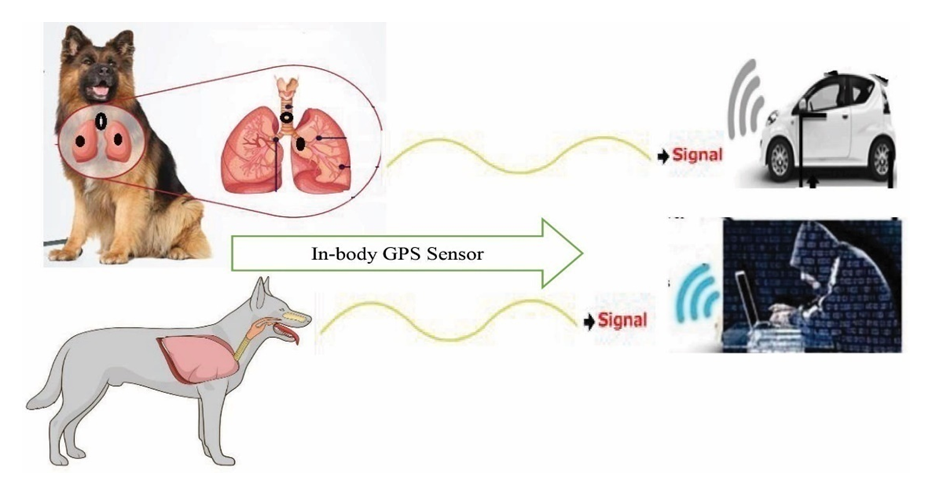 | Figure 4. Tracking towards Lungs of dog with In-body wireless GPS sensor at a fixed location |
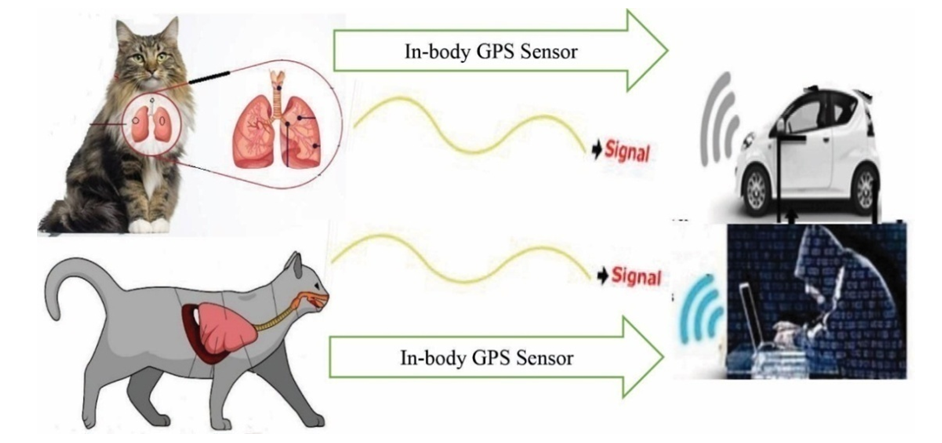 | Figure 5. Tracking towards Lungs of cat with In-body wireless GPS sensor at a fixed location |
2.6. Data Compilation and Analysis
- All quantitative and qualitative related experimented data were collected and compiled according to research objectives. These compiled data checked for accuracy from diverse sources are also verified for the preparation of master sheet for analysis and interpretation using update software like MS Office 2021, R ver. 3.6 and SPSS ver. 27.
3. Results
3.1. Identified Symptoms
- Misuse of advanced wireless sensor devices at a specific GPS location, depending on the underlying cause, has led to sudden onset of acute respiratory syndrome in a variety of situations and with a variety of symptoms, such as:ü Sudden extreme effort to breatheü Unusual coughü Frequent sneezingü Discharge from the noseü Sudden feverü Hypnosisü Unexpected sweatingü Severe lung infectionü Lung injury due to high radio frequency abuseü Sudden serious illnessü Sudden runny noseThe tracking time showed in Figure 6 mentioning Feline Body Mass Index occurring sudden acute respiratory distress syndrome. Excess weight animals affected quickly in SARDS than that of other animals. The study evaluated the condition of cats and dogs and cured them through emergency treatment at once. A thorough history of cat and dog health, the onset of symptoms, and possible events prior to this condition was recorded, such as injury to any part of the body, or inhalation of gas, sensor smoke, or solids. In addition to emergency treatment, the researcher was able to find the underlying cause of sudden lung failure.Blood test, urine test, serum biochemical test and blood gas analysis were normal. One of the most important diagnostic methods used in veterinary practice to diagnose SARDS is blood gas analysis. Researchers have instructed X-rays and echocardiography of the chest to visually examine and evaluate the functioning of the lungs and heart.
3.2. Impact on Wireless Sensor Tracking
- The impact of wireless sensor tracking included different steps, which as shown in Figure 6.
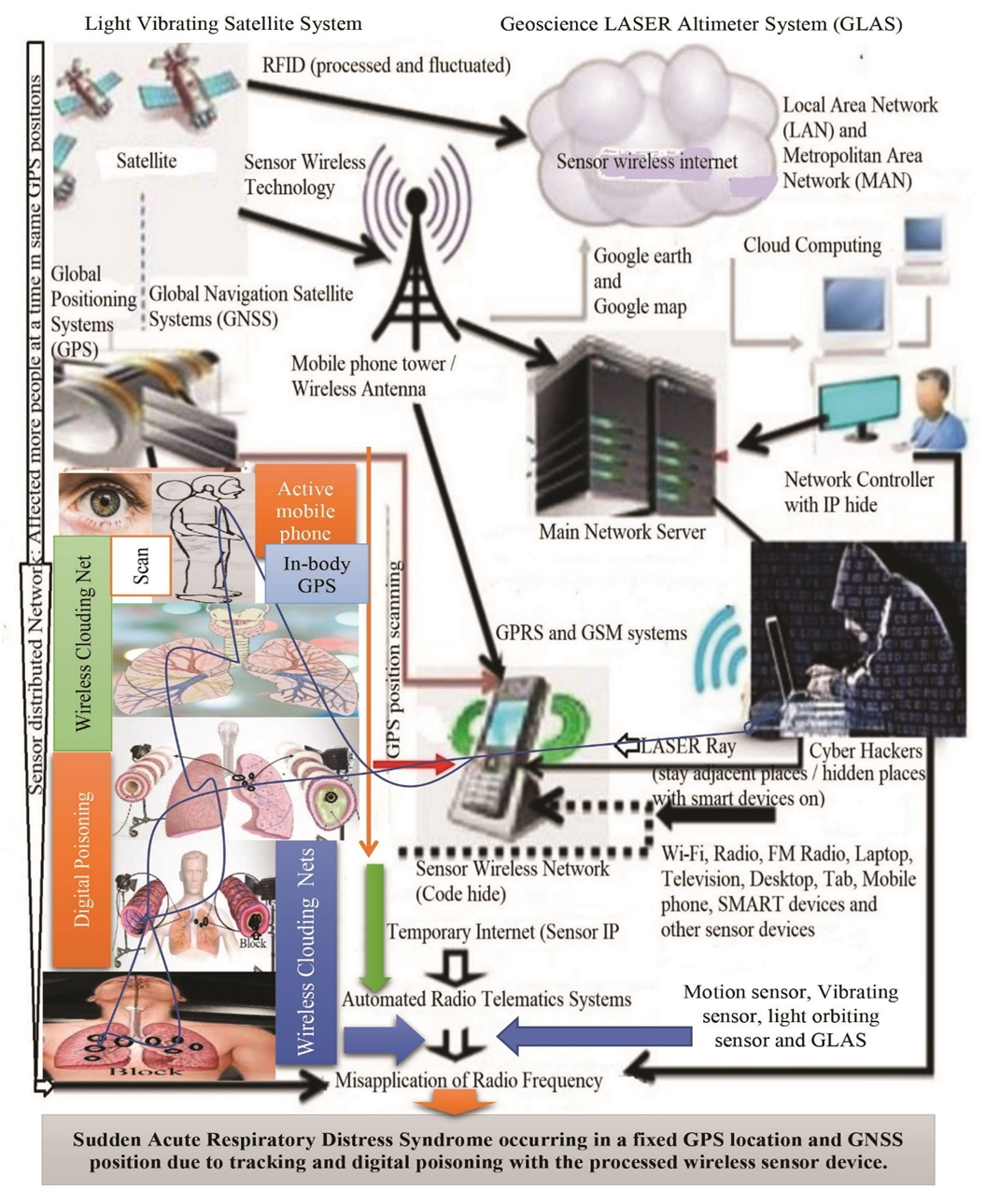 | Figure 6. Impact of advanced wireless sensor tracking towards lungs |
3.3. Tracking Time
- Excess weight individuals affected in SARDS in less time than that of other FBMI, which as shown in Figure 7.
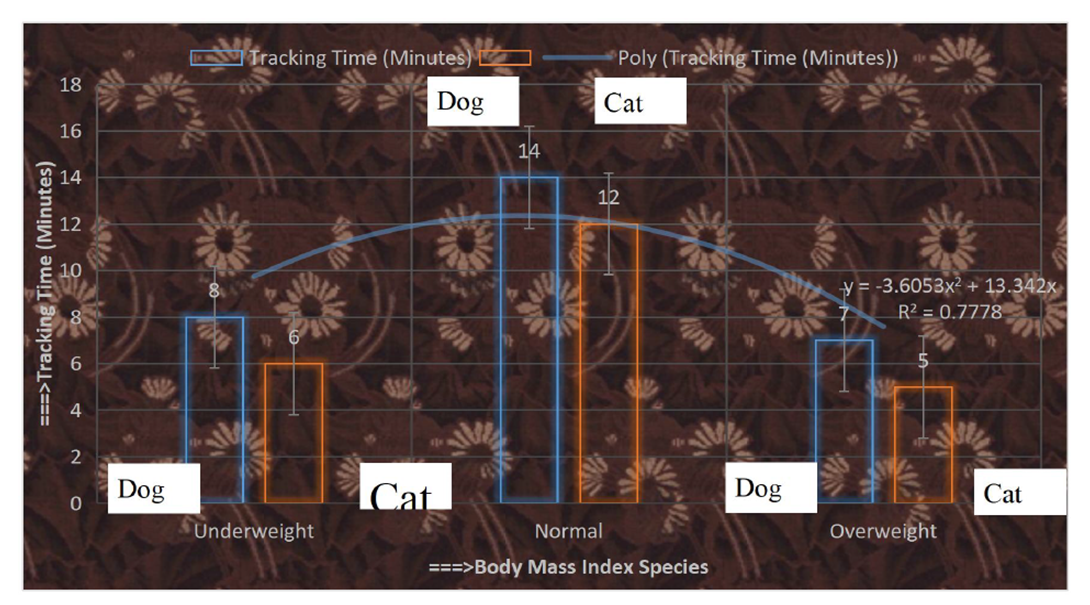 | Figure 7. Tracking time towards animals with FBMI |
3.4. Risk Factors
- Most people who develop SARDS were already hospitalized for another condition and many are critically ill. The patients are specially at risk if they have a widespread infections and disorders in sepsis. The individuals who have a history of chronic alcoholism are at higher risk of developing SARDS. They are also more likely to die of SARD due to collapsing lung through tracking with smoke sensor, which as shown in Figure 8 and Figure 9.
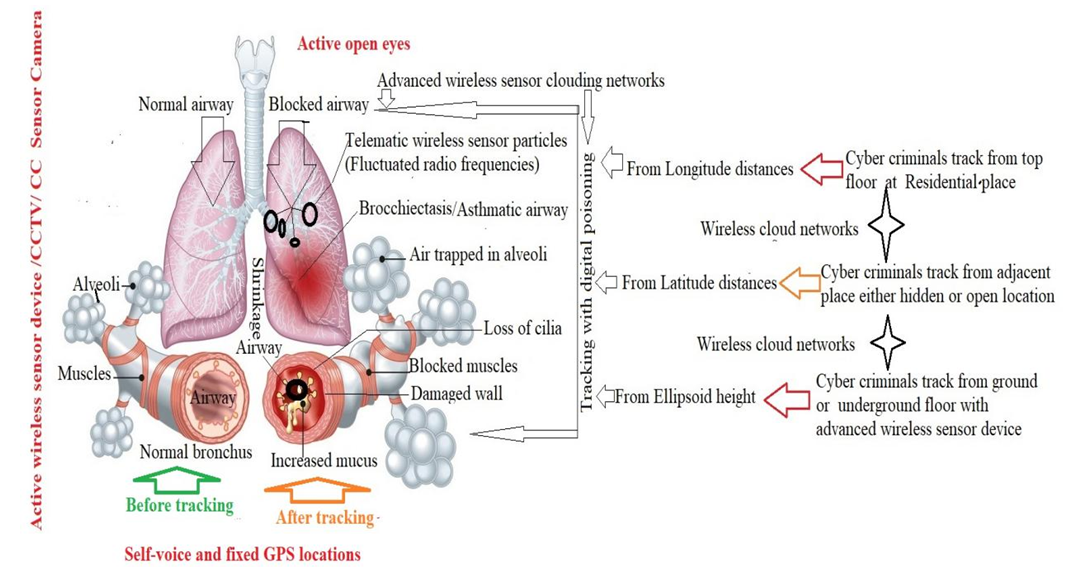 | Figure 8. Sudden onset airways block due to tracking with wireless sensor |
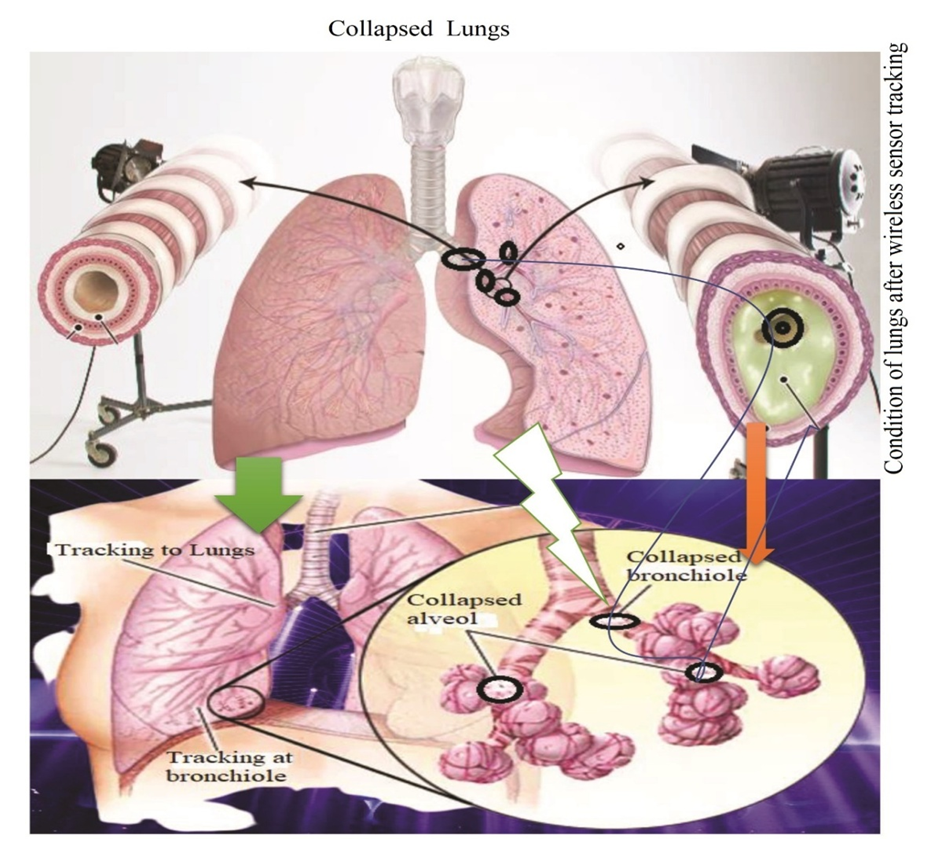 | Figure 9. Collapsed Lungs due to misuse of wireless sensor tracking |
3.5. Complications
- Due to misuse of wireless in-body GPS sensor, there are several complications in this study. If the patients have SARDS, they develop other medical problems while in the hospital. The major complications were identified from the study, such as: Lying still in the hospital while the patients were on a ventilator increasing their risk of developing blood clots, particularly in the deep veins in their legs. If a clot forms in their legs, a portion of it broke off and travelled to one or both of their lungs (pulmonary embolism)- where its blocked blood flow. In most SARDS cases, a breathing machine called a ventilator used to increase oxygen in the body and force fluid out of the lungs. However, the pressure and air volume of the ventilator could force gas to go through a small hole in the very outside of a lung and caused that lung to collapse. The ventilator is attached directly to a tube inserted in patient’s windpipe, which made it much easier for germs to infect and further injure their lungs. Scarring and thickening of the tissue between the air sacs occurred within a week of the onset of SARDS. This stiffened their lungs, was making it even more difficult for oxygen to flow from the air sacs into their bloodstream. The airways block due to tracking with the wireless in-body GPS sensor, which as shown in Figure 10.
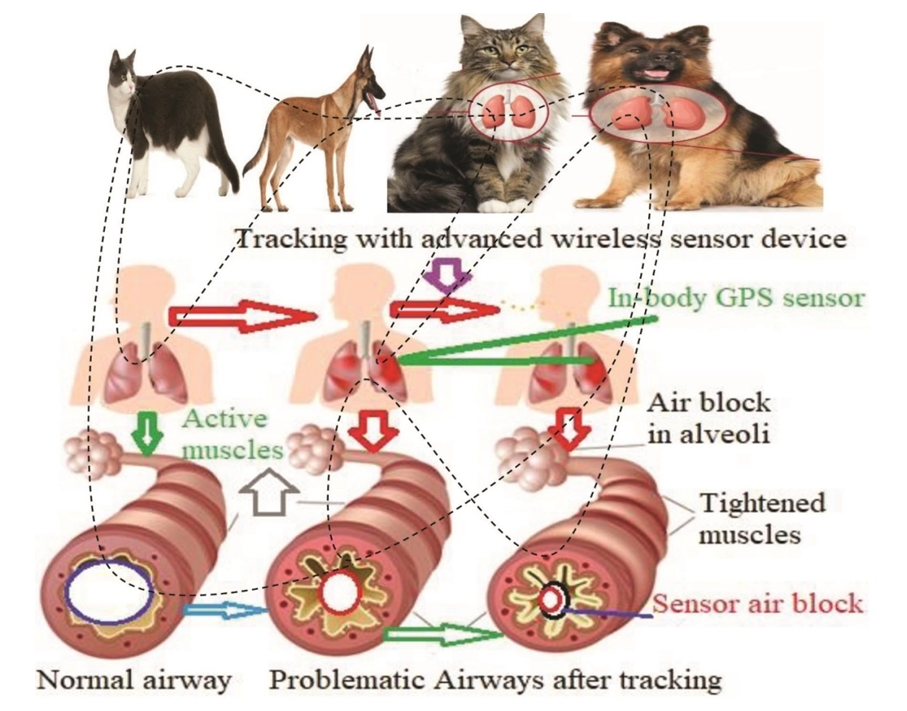 | Figure 10. Problematic airways due to tracking with advanced wireless sensor devices |
3.6. Inference
- The results of the above study show the wireless sensor network tracking is the root cause of SARDS in humans and animals, instantly making them sick and killing them at a fixed GPS location due to active open eyes and uttering self-voice. Therefore, use of advanced technology, safe measures and dynamic law enforcement with RAFAC (Rapid Action Force Against CASSID) linking global public health security.
4. Discussion
- The study illustrates with different parameters according to its objectives on sudden acute respiratory distress syndrome (SARDS). The SARDS is a part of CASSID (Common Acute Sensor Sudden Infections and Disorders) on medical emergency that requires special attention for immediate treatment. Infected cats and dogs in this study require emergency treatment in an intensive care unit with wireless network isolator and anti-radiation unit. In addition to emergency treatment, the underlying cause was of course established and prompt DRAST (Disease Recovery through Advanced Sensor Technology) treatment was arranged to avoid further complications or death [185,196].
4.1. ARDS and Technology
- Despite recent advances, treating SARDS in health practice is one of the most difficult and challenging issues. Supplemental oxygen therapy was started immediately to reduce shortness of breath. If the experimented cat and dog do not respond well to oxygen therapy and continue to have severe shortness of breath, ventilator-assisted breathing may be more successful [231-235]. Medications to treat SARDS include antibiotics, analgesics, fluid therapy, and corticosteroids to reduce inflammation and swelling. Frequent readings of temperature, pulse, respiratory rate, and blood pressure are required to follow the cat's progress in the early stages of treatment [236-240]. If cats and dogs are kept on ventilator support, regular physiotherapy sessions and frequent changes in body position are required to avoid complications related to ventilator support. Cats and dogs infected with SARDS are kept in strict cages and kept on a wireless network until fully recovered [182,183]. Many people think that shortness of breath means asthma [241-242]. But not all shortness of breath is asthma. Asthma causes special types of shortness of breath. It starts suddenly. Then there is a sound like a flute inside the chest. At the same time coughing and shortness of breath are felt in the chest. In addition to asthma, heart disease can also cause shortness of breath. The left side of the heart becomes useless but there is severe shortness of breath. Its name is cardiac asthma. This is because of the accumulation of water in the lower part of the lungs. The patient cannot lie down with so many problems, it only increases when he is sick. Asthma of both the lungs and the heart there is shortness of breath in both cases. The doctor can easily tell by the patient's age, symptoms and chest examination what kind of asthma the patient is suffering from. In addition, kidney failure can also cause shortness of breath [243-245]. Difficulties in various organs also cause shortness of breath [246-250]. Because the causes of these shortness of breath are different, the treatment system is different [251-261].
4.2. Modified Definition of SARDS
- Any lung problem or disease can cause shortness of breath. The main symptom of pneumonia is shortness of breath. The more parts of the lungs are affected, the more obvious the shortness of breath will be. Chronic bronchitis also causes shortness of breath. Among the various causes of this disease are excessive smoking, dusty and smoky environment and in some cases hereditary causes. Lung asthma has many similarities with chronic bronchitis. Although these two diseases are of completely different types and natures [262-272]. Shortness of breath in chronic bronchitis continues to increase day by day. The earlier definition of ARDS as: ARDS is an acute diffuse, inflammatory lung injury, leading to increased pulmonary vascular permeability, increased lung weight, and loss of aerated lung tissue…[with] hypoxemia and bilateral radiographic opacities, associated with increased venous admixture, increased physiological dead space and decreased lung compliance [229]. There is disagreement among doctors, nurses and others about the current definition of SARDS. The disease is a component of CASSID (Common Acute Sensor Sudden Infections and Disorders), which is caused by sudden sensor poisoning. Cyber trackers can kill selected people and animals via instantaneous wireless sensor tracking in a GPS designated area, where health professionals can easily prevent disease through DRAST (disease recovery through advanced sensor technology) systems. But many physicians, scientists and researchers do not know yet. The current definition of ARDS is very old. Depending on the long time and the discovery of advanced technology, there is no similarity in definition with the symptoms of this disease. Therefore, the World Health Organization (WHO) can redefine the disease as a leading body with the participation of medical experts, sensor technologists and relevant authorities from around the world. However, in this case, this study may serve as an aid.
4.3. SARDS and Chronic Conditions
- Sudden head, chest, other major injuries, falls or car accidents, etc., can directly damage the lungs or parts of the brain that control breathing [186-188]. People with severe COVID-19 may have SARDS [190-196]. It also develops pancreatitis (inflammation of the pancreas), extensive blood circulation and burns. The most common cause of SARDS is sepsis, which is a serious and widespread infection of the bloodstream [273-275]. Inhalation of harmful substances, inhalation of smoke or high concentrations of chemical fumes can result in SARDS, such as inhaling (desirable) vomiting or almost drowning. SARDS occurs when fluid accumulates in a tiny and elastic air sac (alveoli) in the person's lungs. The fluid prevents their lungs from filling with enough air, which means less oxygen reaches their bloodstream [276-279]. This deprives the individual's organs of the oxygen they need to function. SARDS usually occurs in people who are already seriously ill or have significant injuries. Acute shortness of breath - the main symptom of SARDS - usually creates within hours to days of the injury or infection [280]. Many people who evolve SARDS do not survive due to avoid risks [281-282]. The risk of death increases with age and severity of illness. Some survivors of SARDS recover completely while others experience chronic damage to their lungs [283-285].
4.4. ICU Admission and Healthcare Technology
- Patients with the acute respiratory distress syndrome (ARDS) are characterized, to different degrees, by an alteration in pulmonary endothelial and epithelial permeability with associated lung edema [59]. Acute circulatory failure is highly prevalent and potentially prognostic in ARDS patients [286-290]. Optimal fluid management in these patients remains challenging and controversial because it should provide an adequate oxygen delivery while avoiding inadvertent increase in lung edema, thus balancing a liberal versus a restrictive fluid strategy approach. Positive fluid balance and low serum albumin concentration have been found to be independent risk factors for ARDS development [291]. Moreover, an increased body weight related to cumulative fluid balance has been associated with a worse outcome [293]. Clinical data from patients with ARDS confirm that fluid overload is deleterious for patient outcomes. Early in the course of critical illness, positive fluid balance prior to the development of ARDS portends its development and a higher risk of dying [189]. Net positive fluid balance occurs in the majority of patients at the onset of ARDS even when closely monitored, and predicts prolonged mechanical ventilation, longer intensive care unit (ICU) and hospital stay, and higher mortality [294],[295].
4.5. Focus on Data Richness
- Increased hydrostatic pressure is common in ARDS patients, at least intermittently during the course of the disease, and associated with a higher risk of death [296,297]. Intravenous fluid administration is necessary in many critically ill patients, and the ebb and flow phenomenon across time in these patients is evident in observing the lowest mortality for sepsis and ARDS patients receiving adequate early fluid administration followed by later conservative fluid management, compared to patients who received either inadequate early fluids or more liberal later fluid administration [298,299]. Compared to healthy subjects, ARDS patients present a higher amount of extravascular lung water (EVLW) for a given arterial pulmonary pressure and a linear increase in the fluid shift from capillaries to alveoli consequent to any increase in the pulmonary pressure [300],[301],[302].
4.6. Research Potentiality
- Research into wireless sensor technology has proven to be revolutionarily beneficial to mankind over the past few decades, but due to the lack of proper security, its misuse is also increasing day by day and cyber criminals are emerging new pandemics [303],[304].
4.7. Diagnosis and Treatment
- Pathological features, advance sensor technology, clinical presentation, long-term prognosis, complications and major risk factors for SARDS vary abruptly between different CASSID diseases as a result of being in a cloud network system, which lead to certain deaths. In this moment all types of medicine can’t recover from SARDS due to switch-on at tracking wireless sensor device. So, PANCU (Personal Area Network Control Unit) and tightly closed eyes are essential for life safety net [309].
4.8. Innovation
- The lungs are an important part of the human body used for breathing. People die when this breathing stop. Both natural and technological stoppage can occur at a specific GPS location. But technological stoppage occurs sudden due to tracking with advanced wireless sensor devices. Many people are dying everyday in the world due to sudden cessation of breathing. But many of us do not know the secret of the sudden cessation of breathing? Even many scientists and researchers do not know the exact source of this SARDS. But this researcher does believe all mankind will know it from today’s published research paper through diverse media.
4.9. Legal Aspect in Trust
- Sensor technology sometimes creates a lot of problems in CASSID that may be subjected our life to be lost. The victimization of such deed is not only any individual but also national, regional and global levels, which as shown in Figure 11. Some legal aspects in trust are outlined to tackle this sensor health crime, particularly in SARDS, such as:i. Public Awareness or Social Movement: Social movement should be launched and public awareness should be raised against these crimes through the media of press, TV, newspaper and other audio-visual aid. Seminar, symposium etc. should be arranged to aware the public.ii. Invention of New Technology: Modern and sophisticated devices should be invented as well as upgraded to identify such incident. Adequate training should be provided to the law enforcing authority. iii. Amendment of Law providing Vehement Punishment: The Digital Security Act 2018 needs to be amended and should incorporate vehement punishment for the violator who commits crime against human body using sensor technology.iv. Incorporation of Healthcare Education to Curriculum: Healthcare Education should be incorporated to the curriculum of every disciplines so that the learners can be acquainted and be aware of the bad impact of cyber technology.v. Monitoring system: Proper monitoring is perquisite to such types of crime. Lack of vigilance paves the criminal a way to commit crime. So, the offenders should always be monitored to curb this crime.vi. Global Effort: The problems is not confined to the territory of any individual state rather it is a global problem. So, pragmatic approach and effort of the whole world needs to be taken.
 | Figure 11. Legal aspects in Trust for sustained life |
4.10. Challenges
- We are still investigating the dynamic security measures of the proposed public health policy to address real-world challenges. This study is a challenging step in image processing, which plays a key role in identifying humans, animals and objects, and their patterns [305,306,307,308]. On the other hand, after the publication of this study, many researchers, scientists, health professionals and others will be misled by cyber criminals in various ways through message bounce, scamming text, phishing voice and false interface. As a result, cybercriminals will infect the readers through CASSIDs by keeping their eyes open, talking through their mouths, and having a specific GPS location. However, the conscious community will be fully aware of this and will not be disappointed, as opportunities for cooperation between the World Health Organization (WHO), INTERPOL and RAFAC (Rapid Action Force against CASSID) will increase the security assistance to victims as citizens of UN member states.
4.11. Paths Forwards
- If the SARDS patient is taken to the hospital's IUC (Intensive Care Unit), the patient is at greater risk if the ICU does not have a wireless network control unit. Since the patient's bed is in a specific GPS location, cybercriminals make it even sicker by cloud-tracking around the hospital. This cloud wireless network disrupts the flow of oxygen through digital poisoning and is virtualized by wireless telematic particles in the patient's lungs, and cybercriminals increase the amount of radio frequency to further complicate the patient's condition. When this cloud wireless network applied to the patient is connected, the amount of digital poisoning due to the obstruction of oxygen flow increases, more mucus is produced instantly and the primary alveoli are blocked by digital knots. Eventually, the patient died instantly due to severe shortness of breath. Again, wireless digital gravity fluctuates body-temperature and blood pressure but causes sudden shortness of breath, which is blocked by node tracking for one person and re-tracking is distributed to multiple people to block the respiratory system at selected GPS locations. In this case, the patient cannot be cured by various high quality medicines, treatment by an experienced doctor or advanced care by a nurse. Therefore, the first and foremost task of such patient recovery is to treat the patient through an 'anti-radiation zone' and a private network control unit. People are responsible for SARDS and people know how to cure this disease. This requires participation, cooperation, integration and awareness of all. Global health security can be ensured through timely government effective legislation, administrative support from higher authorities, coordination of development agencies and public participation in the media.
5. Conclusions
- In concluding, the ARDS occurs through the misuse of advanced wireless sensor technology due to active open eyes, self-voice and a specific coordinate. For this reason, SARDS is a very serious health problem that requires endless proper awareness for successful treatment, dynamic management and effective healthcare of the condition. The patients must follow the doctor's instructions closely and they consult their doctors if they have any doubts. If SARDS is not finally diagnosed and resolved, another occurrence of shortness of breath may follow. The patients who have survived in SARDS usually need time, rest and good nutrition for a full recovery. Patients should not be confined to crowded or hot places and they have enough time to walk or exercise. Even after the condition has resolved, many patients may have scarring in the lungs, a condition called fibrosis, and the lung tissue may become stiff and unable to hold oxygen. It is therefore very important to follow the diet and direction made by the physician and adhere to the best measures to prevent recurrence to keep the activity to a minimum. Lastly, this is a very timely study- it will benefit those around the world who take it. There is no doubt about this research, but cyber criminals can create suspicion among people. So, everyone should be aware of this research.
6. Declaration
- FundingThis research work is a part of PhD Thesis, which was funded by the Zamalah Postgraduate Scholarship of UNIMAS, Malaysia and also sponsored by the Information and Communication Technology Division, Ministry of Posts, Telecommunication and Information Technology, Government of People’s Republic of Bangladesh. The funders had no role in the design of the research, in data collection, analyses or final interpretation of data, in the writings of the manuscript, or in the decision to publish the findings.Data AvailabilityThe data being used to support the findings of this research work are available from the corresponding author upon request. Competing InterestsThe authors declare no potential conflict of interests in this research work.
ACKNOWLEDGEMENTS
- The authors acknowledged the authority of Universiti of Malaysia Sarawak (UNIMAS), Malaysia for providing the Zamalah Postgraduate Scholarship for the completion of PhD degree. The authors are also grateful to the authority of the Information and Communication Technology Division, Ministry of Posts, Telecommunication and Information Technology, Government of People’s Republic of Bangladesh, for PhD Fellowship during the higher study in Malaysia. The authors acknowledged the authority of North East Medical College & Hospital, affiliated with Sylhet Medical University, Sylhet, Bangladesh for kind supports.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML