-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(6): 602-605
doi:10.5923/j.ajmms.20221206.02
Received: May 8, 2022; Accepted: May 27, 2022; Published: June 2, 2022

Our Experience in Studying the Effect of a Genetically Modified Products on the Colon Microflora Laboratory Animals
Karimova Maksuda Ahmedjanovna1, Matnazarova Gulbaxor Sultanovna2, Avozmetov Jasur Egamberganovich3
1Assistant of the Department of Microbiology of the Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan
2Doctor of Medical Sciences, Head of the Epidemiology Department TMA, Tashkent, Uzbekistan
3Department of Pediatric Surgery, Anesthesiology and Resuscitation, Urgench, Uzbekistan
Correspondence to: Karimova Maksuda Ahmedjanovna, Assistant of the Department of Microbiology of the Urgench Branch of the Tashkent Medical Academy, Urgench, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of the experiment In the experiment, GM-soy was used to determine the extent of exposure of white non-white rats to colon microbiocenosis. It was found that in the normal microflora of the colon of rats fed with GM-free soybeans, different reliable quantitative differences were found compared to intact laboratory animals Bifidobacterium spp (1.28-fold decrease), Lactobacillus spp (1.53-fold decrease), Enterobacter spp and Proteus spp (4.1 and an increase of 6.25 times). This did not indicate the development of complete dysbiosis, as no intergroup distinction was found between lactose-negative and lactose-positive strains of Escherichia coli. While GM-shadow-fed laboratory animals contained all 5 cited elements of dysbiosis, they did not show up clearly in rats consuming GM-shade. No signs of dysbiosis were detected in intact laboratory animals, dysbiosis symptoms were poorly developed in GM-free soy feeding (DI I), and dysbiosis symptoms were evident in GM-shadow-fed animals (DI II).
Keywords: GMO soy, White outbred rats, Microbiocenosis of the large intestine, Intestinal dysbiosis, Dysbacteriosis index
Cite this paper: Karimova Maksuda Ahmedjanovna, Matnazarova Gulbaxor Sultanovna, Avozmetov Jasur Egamberganovich, Our Experience in Studying the Effect of a Genetically Modified Products on the Colon Microflora Laboratory Animals, American Journal of Medicine and Medical Sciences, Vol. 12 No. 6, 2022, pp. 602-605. doi: 10.5923/j.ajmms.20221206.02.
1. Introduction
- In addition to the various microorganisms that enter the body from the external environment, there are also microorganisms in the human body that live in symbiosis with it and form the normal human microflora. They are located in different biotopes and are important for the functioning of the organism. One such biotope is the colon, whose normal microflora, consisting of indigenous and facultative microorganisms, is essential for life activity. It is known that the microbiocenosis of the colon consists of more than 450 microorganisms and is involved in the metabolism of the "host" organism and the formation of colonization resistance in the intestine.Disruption of the normal microflora of the colon under the influence of various external and internal factors is characterized by a qualitative and quantitative imbalance of the indigenous and facultative microflora in it and is called intestinal dysbiosis. Many physical, chemical, and biological factors can be examples of factors that lead to intestinal dysbiosis.Today, a lot of scientific work has been done on the different effects of genetically modified (GM) products on the human body, and experts are divided on this issue, along with the opinion that these products have no adverse effects on the human body [2,10]. There are many proven works [37,9]. Subsequent scientific studies have shown that GM products have a negative effect on the immune system [1], liver and pancreas [8], thymus and spleen [11], as well as hematological, biochemical changes, mutagenic and reproductive activity [5,6], there are studies showing that it has a negative effect on bone marrow cells [12].An analysis of many scientific literatures shows that there are few studies to determine the degree of impact of GM products on the microbiocenosis of human biotopes, including the microbiosis of the colon, and they are scattered.In view of the above, the aim of the study was to determine the degree of effect of GM-shadow on the microbiocenosis of the colon in white rats in the experiment.
2. Materials and Methods
- To study the effects of GM products on the body obtained using new technologies, experimental studies were conducted in laboratory animals (white rats). For this purpose, we used soy flour (GM-product) grown abroad.For this purpose, a total of 90 male white rats were involved in the study, which were divided into 3 groups: Group 1 - intact white rats with a standard vivarian diet, not fed with GM or without GM (n = 30); Group 2 - white non-GM rats included in the standard vivariate diet without GM (n = 30); Group 3 - white pedigree rats fed with GM-shade on a standard vivariate diet (n = 30).These groups were representative and differed from each other by only one character. Emphasis was placed on whether the studies were randomized and that principles of evidence-based medicine were followed. The study strictly adhered to the ethical principles and biological safety rules of working with laboratory animals [4].After the white mass of rats was delivered to the bacteriological laboratory, bacteriological examinations were performed using Bergy's Manual Systematic Bacteriology (1997) using appropriate nutrient media (Blaurokk, SRM-4 (MRS-4), Endo, Saburo media, egg yolk agar, etc.). microorganisms were identified and differentiated: Bifidobacterium spp, Lactobacillus spp, Escherichia coli, Enterobacter spp, Proteus spp, Staphylococcus spp, Streptococcus spp, Candida spp. Intergenerational and interspecific identification was performed using food media from HiMedia (India).Statistical processing of the results was carried out using traditional variational statistical methods, and the principles of evidence-based medicine were followed in organizing and conducting the research.
3. Results and Their Discussion
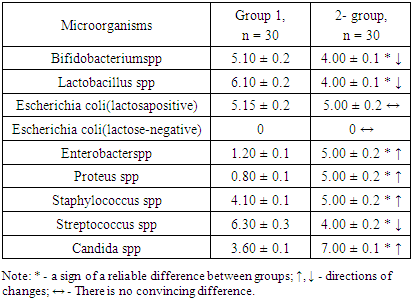
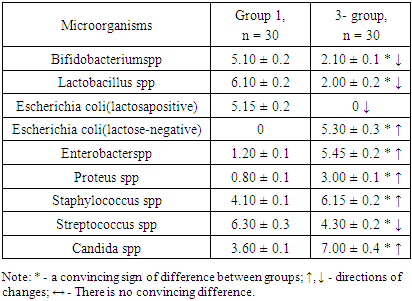
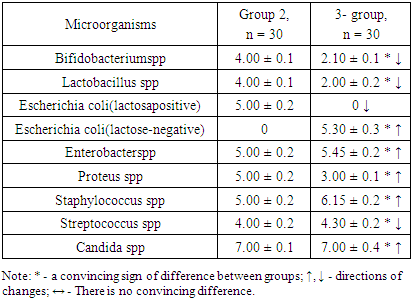
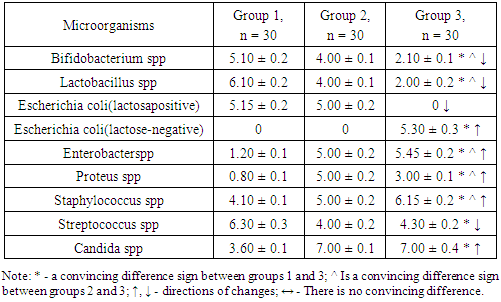
- The results obtained showed that there were convincing differences in the quantitative indicators studied between the groups being compared (Table 1).
|
|
|
|
4. Conclusions
- 1. GM-free soy-fed white rats in the normal microflora of the colon compared with reliable intact laboratory animals Bifidobacterium spp (1.28-fold decrease), Lactobacillus spp (1.53-fold decrease), Enterobacter spp, and Proteus spp (4, An increase of 16 and 6.25 times, respectively). These are the initial signs of dysbiosis and do not indicate the development of complete dysbiosis, as no group differences between lactozanegative and lactosapositive strains of Escherichia coli have been identified.2. Quantitative indicators of Bifidobacterium spp and Lactobacillus spp in laboratory animals fed with GM-soy were significantly reduced by 2.43 and 3.05 times compared to intact rats. This decrease was an external factor negatively affecting them, and in this experiment it was interpreted as a GM-soy. This condition was interpreted as the first element of dysbiosis formed in the colon biotope.3. In contrast to intact ones, white lactating rats fed with GM-soy did not produce lactose-negative Escherichia coli, while lactose-positive Escherichia coli did not produce flour, and vice versa. Lactation of lactosanegative strains, the absence of lactose-positive strains, has been shown to be a second element of colonic dysbiosis.4. In laboratory animals fed with GM-soy, Enterobacter spp and Proteus spp were found to be 4.54 and 3.75 times higher, respectively, than in the control group, proving to be the third element of colon dysbiosis.5. No significant changes in gram-negative cocci were observed in elements 1-3 of colonic dysbiosis with obvious manifestations of this condition - Streptococcus spp in the main group decreased 1.47 times compared to intact laboratory animals, coagulazapositive Staphylococcus spp 1.50 times more reliable. increased significantly. This intergroup incompatibility was interpreted as the fourth element of colon dysbiosis.6. The quantitative index of Candida spp in white-bred rats fed with GM-soy was significantly increased by 1.94 times compared to those not fed with this product, as the fifth element of colon dysbiosis.7. In laboratory animals fed GM-soy, all 5 of the cited elements of dysbiosis were present, whereas in rats consuming soy without GM, they were not evident.8. Determination of dysbacteriosis index, indicating the I and II degrees of dysbacteriosis, gave the following results: in group 1 - 0.31 <0.1 (DI I); 0.37 <0.5 (DI II); In group 2 - 0.38 <0.1 (DI I); 0.77 <0.5 (DI II); In group 3 - 1.29 <0.1 (DI I); 3.56 <0.5 (DI II). There were no signs of dysbiosis in intact laboratory animals, the symptoms of dysbiosis were poorly developed in GM-free soy feeding (grade I), and the symptoms of dysbiosis were pronounced in GM-soy-fed animals (grade II).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML