-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(5): 595-598
doi:10.5923/j.ajmms.20221205.29
Received: Apr. 19, 2022; Accepted: May 13, 2022; Published: May 27, 2022

Pharmacological Correction of Cytolytic Syndrome in Isoniazid-Induced Acute Hepatitis
Khudayberdiev Kh. I.
Department of Pharmacology, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Khudayberdiev Kh. I., Department of Pharmacology, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The comparative hepatoprotective activity of lesbokhol, celagrip, konvaren and legalon was studied in experiments on 30 white outbred male rats weighing 180–200 g. The acute toxic hepatitis was induced by the orally administration of the anti-tuberculosis drug (АTD) - isoniazid at a dose of 70 mg/kg. The effectiveness of the investigated medicines lesbokhol (25 mg/kg), celagrip (25 mg/kg), convaren (50 mg/kg) and legalon (100 mg/kg) in eliminating the cytolytic syndrome was established. The cytolysis markers activity of the alanine aminotransferase (ALT, in IU/l) and aspartate aminotransaminase (AST, in IU/l) was determined in blood. there was an increase in ALT activity by 150.6% and AST by 36.7% under the influence of isoniazid in rats. The activity of ALT in comparison with the control group was lower by 33.5% in the group of animals treated with lesbokhol and by 33% in the group of animals treated with legalon. Along with this, in the groups of animals treated with lesbokhol, celagrip, convaren, and legalon, the activity of AST in the blood serum also decreased compared to the control group by 15.5%, 21.7%, 13.5%, and 11.9%, respectively. With respect to the low toxicity of Celagrip and its higher hepatoprotective activity, we consider the possibility of using it in patients with tuberculosis in the treatment of drug-induced hepatitis.
Keywords: Toxic liver damage, Anti-tuberculosis drugs, Lesbokhol, Celagrip, Convaren
Cite this paper: Khudayberdiev Kh. I., Pharmacological Correction of Cytolytic Syndrome in Isoniazid-Induced Acute Hepatitis, American Journal of Medicine and Medical Sciences, Vol. 12 No. 5, 2022, pp. 595-598. doi: 10.5923/j.ajmms.20221205.29.
Article Outline
1. Introduction
- Chronic diseases of the hepatobiliary system are the main causes of death among economically developed countries. Annually, 40 million people die from cirrhosis of the liver and hepatocellular carcinoma in the world [1].In addition to viruses, some household chemicals, pesticides, alcohol, industrial substances, and a number of drugs also have a hepatotoxic effect [2].The amount of drugs consumed by the population, the appearance of a new generation of drugs with high pharmacological activity, the irrational use of drugs, medical errors, and the use of counterfeit drugs ultimately lead to liver damage, since the detoxication function of the liver is central to the biotransformation of xenobiotics. About 10–28% of all adverse reactions from the application of pharmaceutics lead to various liver damage, up to fulminant liver failure [3].In recent years, the number of cases of drug-induced liver damage has increased significantly. This problem is faced by physicians of all specialties, and there are some difficulties in diagnosis and treatment in time [4].It is noted that a good clinical effect is not always achieved with the use of legalon (reference hepatoprotector). A decrease in the permeability of cell membranes under the influence of silymarin is associated with stimulation of protein and phospholipid synthesis, which leads to stabilization of cell membranes. As a result, the loss of cell components, including intracellular enzymes - transaminases, is prevented, which is clinically manifested by a decrease in the cytolytic syndrome. In addition, silymarin prevents the penetration of certain hepatotoxic substances, in particular the poison of Amanita phalloides, into the cell. However, the low bioavailability of silymarin when taken orally, the possibility of intensifying the cholestasis syndrome, and proven efficacy only when it is administered intravenously in viral hepatitis C can be attributed to the disadvantages of this group of drugs. [5,6,7]. Therefore, the development of new, effective medicines for the treatment of drug-induced liver damage or the comparative evaluation of known drugs is an urgent problem in pharmacology.The aim of this work was an experimental study of Celagrip, Lesbokhol, Convaren and Legalon on the course of the cytolytic syndrome in drug-induced liver injury.
2. Material and Methods
2.1. Experiments
- The experiment was carried out on 30 outbred male rats in the department of pharmaco-toxicological researches of the Interuniversity Research Laboratory of the Tashkent Medical Academy.Before the start of the study, the animals were kept in a 10-day quarantine, during which the rats were examined and the body weight, behavior, and general condition of the animals were recorded. The main criteria for the inclusion of animals in the study were body weight (not less than 180–200 g); wool cover (smooth and shiny); behavior and general condition (active dynamics of movement and feed consumption). Before the start of the study, rats meeting the inclusion criteria were randomly divided into several groups. The studies were carried out at room temperature 20-22°C.An experimental model of liver pathology was created according to the method of G.N. Mozhokina [8] by administering the anti-tuberculosis drug (ATD) isoniazid at a dose of 70 mg/kg intragastrically for 10 days.In the experiment, the animals were divided into six groups: 1st group — intact rats; 2nd group- control group, animals were received distilled water; 3rd group- rats were received lesbokhol at a dose of 25 mg/kg per os; 4th group- rats were received celagrip at a dose of 25 mg/kg per os; 5th group - rats were received konvaren at a dose of 50 mg/kg; 6th group-rats were received legalon at a dose of 100 mg/kg.The studied medicines were used intragastrically after a 10-day administration of the anti-tuberculosis drug isoniazid. Control animals received distilled water in the same volume. The above-studied medicines were administered once daily for six days.Animals were sacrificed by simultaneous decapitation and blood was taken for biochemical studies. The determination of cytolysis markers (activity of the alanine aminotransferase (ALT, in IU/l) and aspartate aminotransaminase (AST, in IU/l) was included in the complex of biochemical studies. Biochemical blood tests were performed on a Mindray semi-automatic biochemical analyzer (China, 2014) using test kits from Human (Germany) and Cypress Diagnostics (Belgium). Experimental studies were carried out in accordance with the "Rules for Conducting Work Using Experimental Animals", as well as the rules adopted by the European Convention for the Protection of Vertebrate Animals used for experimental studies or for other scientific purposes (ETS No. 123), Strasbourg, 18.03.1986.
2.2. Statistical Analysis
- The data obtained were processed by the method of variation statistics using the paired Student's test and one-way analysis of variance using the standard software package BIOSTAT 2009 with an assessment of the significance of indicators (M ± Std. error). Differences in the compared groups were considered significant at a significance level of 95% p <0.05.
3. Results and Discussion
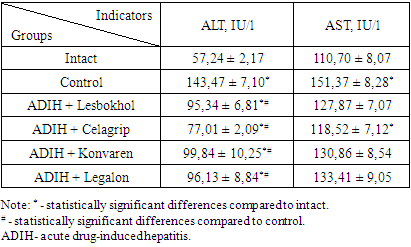
- The choice of isoniazid as a drug for the study was due to the fact that it belongs to the first-line drugs for the treatment of tuberculosis, as well as data indicating that it causes liver damage in 5.4 to 85.7% of patients who receive it for the treatment of tuberculosis [8].The cytolytic syndrome is one of the main reasons leading to the death of hepatocytes or the development of hyperenzymemia. In clinical practice, a biochemical study of the activity of ALT and AST enzymes in blood serum is often used for the evaluation of the level of this syndrome [5].The results of the studies showed that there was an increase in ALT activity by 150.6% and AST by 36.7% under the influence of isoniazid in rats (table 1). An increase in the concentration of these enzymes in the blood indicates a significant increase in the permeability of cell membranes and necrosis of hepatocytes.
|
4. Conclusions
- 1. Subchronic administration of isoniazid into rats causes the development of drug-induced hepatitis, which manifests in the development of the cytolytic syndrome.2. Treatment with celagrip reduces the degree of cytolytic syndrome more than convaren and legalon.3. With respect to the low toxicity of Celagrip and its higher hepatoprotective activity, we consider the possibility of using it in patients with tuberculosis in the treatment of drug-induced hepatitis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML