-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(5): 524-530
doi:10.5923/j.ajmms.20221205.15
Received: April 17, 2022; Accepted: May 6, 2022; Published: May 12, 2022

Influence of the Presence of Myocardial Stunning on the Parameters of Left Ventricular Remodeling and Clinical Course after STEMI
S. R. Kenjaev1, A. K. Koyirov1, U. Sh. Ganiev1, N. M. Latipov1, S. R. Kenjaev2
1Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
2Bukhara State Medical Institute named after Abu Ali Ibn Sina, Bukhara, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to compare the characteristics of the left ventricular remodeling parameters and clinical course after STEMI, depending on the presence of myocardial stunning. Introduction. The presence of myocardial stunning in the area of myocardial infarction is one of the important reasons for the development of left ventricular remodeling, along with the death of cardiomyocytes (necrosis), which requires research in this direction. Material and methods. The study included 75 patients with ST-elevation myocardial infarction. In the hospital period, patients were divided into 2 groups: Group I - with the presence of myocardial stunning (n=45), Group II - no myocardial stunning (n=30). 3 months after ST-elevation myocardial infarction, systolic function and remodeling were assessed and the following adverse events were recorded: all lethal outcomes, repeated myocardial infarctions, hospitalizations associated with the progression of heart failure. Results. Left ventricle dilatation after 3 months was observed among patients with no myocardial stunning. Left ventricle end-diastolic volume, left ventricle end-systolic volume in patients without myocardial stunning significantly increased after 3 months: Left ventricle end-diastolic volume from 144±6.3 to 170±7.3 ml; Left ventricle end-systolic volume from 77±3.8 to 98±4.3 ml (p<0.05). On the contrary, in patients with myocardial stunning, indicators characterizing left ventricle volumes did not significantly increase (p>0.05). The presence of myocardial stunning in the infarction zone has a positive effect on the clinical course of the disease and prevents the development of severe forms of heart failure, reducing mortality and the number of repeated hospitalizations. Conclusion. The presence of myocardial stunning contributes to the restoration of local contractility, preventing the progression of heart failure and a positive effect on the clinical course of the disease and prevents the development of severe forms of congestive heart failure, reducing mortality and the quantity of repeated hospitalizations.
Keywords: Myocardial stunning, Left ventricular remodeling, Ejection fraction
Cite this paper: S. R. Kenjaev, A. K. Koyirov, U. Sh. Ganiev, N. M. Latipov, S. R. Kenjaev, Influence of the Presence of Myocardial Stunning on the Parameters of Left Ventricular Remodeling and Clinical Course after STEMI, American Journal of Medicine and Medical Sciences, Vol. 12 No. 5, 2022, pp. 524-530. doi: 10.5923/j.ajmms.20221205.15.
Article Outline
1. Introduction
- Left ventricular (LV) remodeling after ST elevation myocardial infarction (STEMI) is particularly important for the progression of chronic heart failure (CHF) [1-4]. LV remodeling is a pathophysiological process caused by activation of neurohormonal systems, underlying in the base of natural course of heart failure. The degree of LV remodeling and the heart cavities dilatation has a significant impact on the treatment outcome [4-5]. Structural and geometric changes occurring in the heart after MI were first characterized by M. Pfeffer and E. Braunwald as LV remodeling processes [6]. Ischemic remodeling of the left ventricle most often develops as a result of the cardiomyocytes death at MI and can also be the result of acute ischemia with the development of a "stunned" myocardium. It is known that a "stunned" myocardium is associated with a sharp drop (by 50% or more) of ATP level and suppressed metabolism, the contractility of such a myocardium is significantly reduced. If blood flow in the affected artery is not restored and myocardial oxygen demand is not satisfied, the "stunned" myocardial cells may die due to apoptosis, the so-called "programmed death" of cardiomyocytes. It seems likely that LV injury with the development of "stunned" myocardium, which is present at MI, can lead to significant structural and geometric rearrangement and LV systolic dysfunction [7]. The severity of LV remodeling processes progression also depends on the presence of viable myocardium, which is defined as the phenomenon of "myocardial stunning" ("stunning") that occurs during acute coronary occlusion followed by myocardial reperfusion. Research of R. A. Kloner has shown that even short-term myocardial ischemia lasting 15 minutes or more causes reperfusion changes in the myocardium ("stunning"), contributing to the development of prolonged post ischemic myocardial contractile dysfunction [8]. At repeated ischemic attacks, the development of reperfusion syndrome leads to repeated "stunning" of cardiomyocytes and ends with irreversible changes in cardiomyocytes or their necrosis. Stunning may also be the cause of ischemic cardiomyopathy, in which repeated episodes of myocardial ischemia development and reperfusion play a crucial role. In addition, there is an evidence that the phenomena of "stunning" and "hibernation" are two sides of the same coin. It has been shown that "hibernation" may be the result of repeated episodes of "stunning", which, as they accumulate, can cause severe ischemic dysfunction and myocardial remodeling.The preserved viable myocardium plays an important role in the development of post infarction remodeling. According to L. Bolognese et al. (2002), after percutaneous coronary intervention (PCI), unfavorable LV remodeling occurred in 30% of patients with ST-segment elevation acute coronary syndrome [9]. This index approached 34% in patients after systemic thrombolysis. The frequency of heart remodeling in individuals with a viable myocardium in the area of ischemic lesion at anterior and posterior localization of AMI was approximately the same [10].At the same time, the absence of a viable myocardium determines the range of patients with AMI who are prone to favorable remodeling outcomes. So, it was shown in a study of L. Bolognese and G. Cerisano (1997) that in patients without a viable myocardium in the area of ischemic lesion, higher volumetric indices of intracardiac hemodynamics were noted after six months [11]. Against the background of intravenous infusion of dobutamine, used to indirectly detect viable myocardium, changes in the index of impaired local contractility in the infarct zone and peak values of creatine phosphoate kinase (CPK) were statistically significantly correlated with the degree of increase of the EDV index: -r=-0.66; р<0.000001; r=0.51; р<.00001, respectively. According to F. Nijland et al. (2002), independent predictors of LV dilatation in patients with AMI after reperfusion are the presence of a viable myocardium, the index of local contractility disorders during dobutamine infusion, and the number of pathological Q waves. The absence of EDV increase in the presence of a viable myocardium in the area of ischemic lesion has been kept for three months of observation (p<0.006) [3].Restoration of subepicardial fibers in the areas of transmural lesions contributes to the restoration of function, prevents dilatation and changes in the shape of the left ventricle at a later terms. Improvement of contractile function is recorded in the absence of systolic thickening of the myocardium through the movement of subepicardial fibers inward [12]. Apparently, reperfusion in the early stages of AMI limits the zone of necrosis, contributing to the preservation of myocardial fibers in the border and subepicardial areas.W. G Schmidt et al. who had examined 264 patients with AMI, found a slight improvement of LV systolic function in the reperfused region in the first 3 days and a significant increase in the interval between 3 days and 6 months [11]. The authors showed that in 10 patients from 21 ones there was a delayed recovery of LV myocardial function. The study of perfusion and myocardial metabolism before and after reperfusion therapy in 56 patients with AMI (32 underwent transluminal balloon angioplasty, 24 - conservative treatment) determined a violation of myocardial metabolism, despite the restoration of perfusion in the acute stage, and its gradual improvement with the gradual restoration of asynergic LV segments kinetics. J. Sanchis et al. determined the presence of a "stunned" myocardium in 47 patients with small focal AMI, while in late follow-up LV function was restored, regardless of residual stenosis of the infarct-dependent artery [7]. Various non-invasive cardiac imaging modalities can be used to assess patients in whom determination of viability is an important clinical issue, specifically: dobutamine echocardiography (echo), stress-echo with contrast, SPECT using either technetium or thallium, cardiac magnetic resonance imaging (cardiac MRI), and positron emission tomography (PET) [13-15]. Low-dose dobutamine stress-echocardiography is still an important and inexpensive method for detecting myocardial stunning and can be safely performed after stabilization of the patient's condition on days 3-5 of the disease [16-17].Early reperfusion in acute STEMI limits the development of extensive areas of necrosis and promotes the appearance of myocardial stunning in the infarcted area, which after some time restores contractile function [18]. This process, according to some authors, takes from three to ten days; according to others – from three to six months. Identification of the zone of myocardial stunning in the infarct area during the hospital period is of great importance for predicting the type of left ventricular remodeling and choosing a method for treating the disease [19-21]. Aim to compare the characteristics of the left ventricular remodeling parameters and clinical course after STEMI, depending on the presence of myocardial stunning.
2. Material and Methods
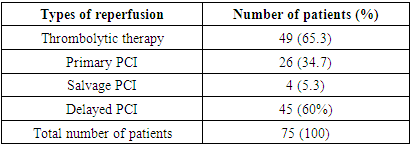
- The study included 75 patients with STEMI aged from 21 to 70 years (mean age was 56±4.3 years) hospitalized in the Department of cardiotherapeutic ICU of the Republican Research Center of Emergency Medicine. STEMI was diagnosed based on the clinical picture and electrocardiographic criteria. Inclusion Criteria: STEMI characteristic clinical presentation and at least one of the following criteria: 1) more than 2 mm ST-segment elevation in two adjacent chest leads or more than 1 mm ST-segment elevation in standard leads; 2) acute left bundle branch block.Exclusion criteria were as follows: • age over 70 years;• pain duration more than 24 hours;• history of myocardial infarction;• severe concomitant somatic diseases (acute cerebrovascular accidents, oncological, mental, surgical diseases) which affect the information content of the study; • objective contraindications for stress-echocardiography; • patients with difficult echovisualization; • patient's refusal to undergo stress-echocardiography. Primary PCI was performed in 26 (34.75%) patients with STEMI. Rescue PCI after unsuccessful thrombolysis within 12 hours was performed in 4 (5.3%) patients, delayed PCI after thrombolysis within 48-72 hours–in 45 (60%) cases. Systemic TLT was performed with streptokinase 1,500,000 IU i/v drip for 45-60 minutes or by an accelerated method - 750,000 IU i/v bolus for 10 minutes (Tab. 1).
|
|
3. Results
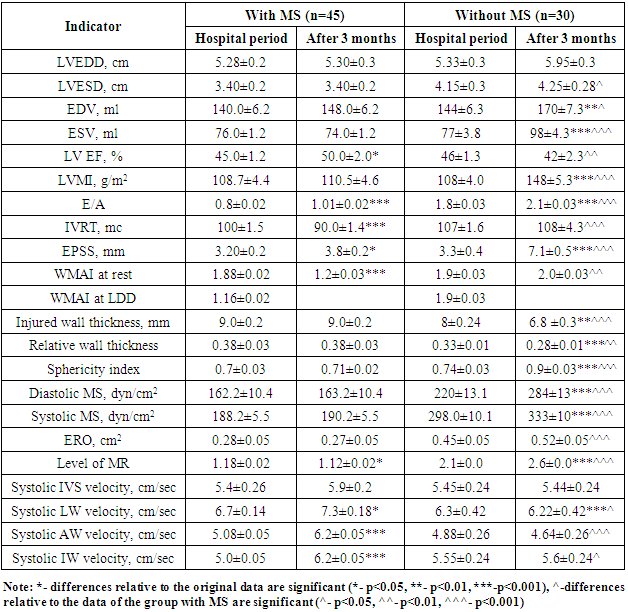
- A decrease of WMAI indicates an improvement in regional LV contractility. WMAI significantly decreased in the group of patients with MS (from 1.88±0.02 to 1.2±0.03), as it was expected from DSE results with low-dose dobutamine in the hospital period. Table 2 shows that the improvement of regional LV contractility was accompanied by an increase of LV EF and a decrease of LVESD and LV ESV (p<0.05). There was an improvement in LV systolic function in the form of a significant increase of LV EF from 45,0±1,2 to 50,0±2,0% in patients with myocardial stunning after 3 months. There was no improvement in regional LV contractility in the form of a decrease in WMAI in patients with no MS after 3 months (initially 1.9±0.03, after 3 months 2.0±0.03), there was a decrease in LV EF from 46±1.3 to 42±2.3% which indicated a progressive decrease in LV contractile function. LV dilatation after 3 months was observed among patients without MS. Volumetric indicators of the left ventricle such as EDV, ESV in patients without MS after 3 months were significantly increased: LV EDV from 144±6.3 to 170±7.3 ml; LV ESV from 77±3.8 to 98±4.3 ml (p<0.05). Although, the indicators characterizing LV volumes were not significantly increased in patients with MS (p>0.05). Indicators characterizing LV remodeling, such as LVMMI (110±4.0 vs 148±5.3 dyn/cm2), sphericity index (0.71±0.02 vs 0.9±0.03 dyn/ cm2), diastolic myocardial stress (163.2±10.4 vs 284±13.0 dyn/cm2) and systolic myocardial stress (190.2±5.5 vs 333±10.1 dyn/cm2) were significantly higher in patients without myocardial stunning after 3 months. This fact shows that the absence of myocardial stunning in patients with STEMI with an initial impaired LV systolic function is an unfavorable sign in relation to the development of maladaptive LV remodeling. In patients with MS we observed the development of an adaptive type of LV remodeling after 3 months. LV dimensions (LVESD, LVEDD), EDV, LVMMI were smaller, there was a trend towards higher EF and lower WMAI. Indicators characterizing the severity of MR also had a negative trend in patients without MS, who showed an increase of ERO (cm2) from 0.45±0.05 to 0.52±0.05. But in patients with myocardial stunning, the transition to the initial value of ERO (cm2) was not observed, on the contrary, there was an unreliable decrease in the degree of MR. The MR index after 3 months in the group of patients with MS was 1.12±0.02, in the group of patients without MS - 2.6±0.02, which was significantly higher in the group of patients with irreversible LV myocardial dysfunction. This fact indicates that mitral regurgitation due to PM dysfunction at AMI in the presence of myocardial stunning will be reversible. The detection of myocardial stunning in the area of the papillary muscle prevents the progression of mitral regurgitation, serious disorders of intracardiac hemodynamics, which is reversible. The walls thickness of the injured area is the main marker for the development of maladaptive LV remodeling. In patients without MS, in whom the infarct zone was completely replaced by myocardial necrosis, hyperechogenicity, thinning and stretching of the injured wall were noted. Wall thinning was not observed in the group of patients with MS. The dynamics of the relative wall thickness significantly differed in the groups after 3 months: in patients with MS after 3 months this indicator was 0.38±0.03, and in patients without MS – 0.28±0.01. The systolic velocity of the walls according to tissue Dopplerography in patients with myocardial stunning increased significantly: in IVS from 5.4±0.26 to 5.9±0.2 cm/s, in LW 6.7±0.14 to 7.3±0.18 cm/s, in AW from 5.08±0.05 to 6.2±0.05 cm/s, in IW from 5.0±0.05 to 6.2±0.05 sm/s. Although in the group of patients with no MS, the systolic velocity of the walls according to tissue Dopplerography tended to decrease: in IVS from 5.45±0.24 to 5.4±0.24 sm/s, in LW from 6.3±0.42 to 6.22±0.42 sm/s, in AW from 4.88±0.26 to 4.64±0.26 sm/s, in IW from 5.6±0.2 to 5.3±0.24 sm/s. As it was predicted by the results of DSE with LDD, there was an improvement in global LV systolic function in the group of patients with MS after 3 months, which was accompanied by an increase in the number of normokinetic segments due to a decrease of hypokinesia and akinesia zones. In 39 (97.5%) patients with MS an adaptive type of LV remodeling was developed. There was no recovery of global LV systolic function in patients without MS, the number of normokinetic segments increased no significantly after 3 months. Also, the number of hypo-, akinetic and dyskinetic segments was not changed significantly, which could be the main reason for the development of maladaptive LV remodeling. 3 months after MI, in patients with MS and adaptive remodeling, the number of normokinetic segments at rest was greater (81% vs 59%, p=0.001), and there were fewer asinergic segments than in patients with dezadaptive remodeling (p=0.001).In patients without MS during the hospital period, the WMAI at rest and against the background of dobutamine was not changed - it confirmed the absence of a viable myocardium. But at adaptive remodeling, WMAI was less than with the desadaptive type (1.5 vs. 2.1), i.e. the degree of violation of local contractility with the adaptive type of remodeling is less, and the geometry of the heart is not changed. 3 months after myocardial infarction, the number of patients with MS decreased due to an increase in the number of patients with restoration of local myocardial contractility function. Analysis of data on the segmental contractility dynamics allows to conclude that the presence of reversible MS contributes to the restoration of local contractility, preventing the progression of heart failure. The detection of MS using DSE is the main indication for performing myocardial revascularization in order to improve the heart contractile function. All patients have received enalapril, carvedilol, aspirin, clopidogrel, spironolactone, atorvastatin for 3 months. The following unfavorable events were recorded after 3 months: all deaths, repeated MI, hospitalizations associated with the progression of heart failure (Fig. 1).
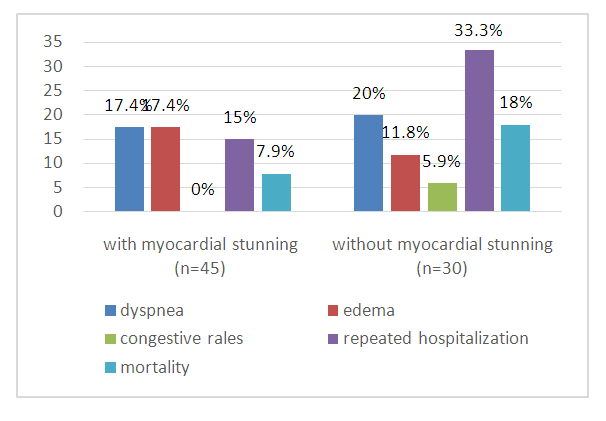 | Figure 1. Analysis of clinical data within 3 months after STEMI |
4. Discussion
- According to this study, the presence of myocardial stunning prevented the development of the heart cavities dilation and the preservation of global and regional systolic function, systolic tissue dopplerography, LV remodeling indicators. It was found that, the severity of hemodynamic shifts was interrelated with the depth and extent of myocardial lesion in patients with acute MI. In patients with the absence of myocardial stunning, the predominance of a huge zone of irreversible myocardial dysfunction leads to the development of a maladaptive form of myocardial remodeling. Discussing the development of remodeling processes mechanisms in patients with acute MI, it should be noted that at the early stages structural and geometric restructuring of the myocardium in the form of LV dilation and hypertrophy is considered as an adaptive reaction and hemodynamic response to LV dysfunction resulting from myocardial injury. However, compensatory mechanisms are inadequate when more than 20% of LV myocardium is affected, which occurs with STEMI. On the other hand, LV dilation can provoke an increase in intraventricular pressure and aggravation of myocardial stress, thereby stimulating further LV expansion and increased myocardial oxygen demand [22-23]. An acute increase in LV wall tension can support a vicious circle, in which high MS induces the processes of maladaptive remodeling with the transition to an increasingly spherical LV shape. The consequence of this process is a further increase of myocardial stress. Also, according to this study, the presence of myocardial stunning in the infarction zone has a positive effect on the clinical course of the disease and prevents the development of severe forms of heart failure, reducing mortality and the number of repeated hospitalizations.
5. Conclusions
- Analysis of data on the dynamics of segmental contractility allows us to conclude that the presence of reversible myocardial stunning contributes to the restoration of local contractility, preventing the progression of heart failure. The presence of MS plays an important role in the formation of late postinfarction LV remodeling types along with other factors.The presence of myocardial stunning in the infarction zone has a positive effect on the clinical course of the disease and prevents the development of severe forms of heart failure, reducing mortality and the number of repeated hospitalizations. The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
List of Abbreviations
- AMI – acute myocardial infarctionCHF - chronic heart failureSTEMI – ST-elevation myocardial infarctionLV - left ventricleEDV – end-diastolic volumeESV – end-systolic volumeLV EF - left ventricular ejection fractionLVMMI - left ventricular myocardial mass indexMS - myocardial stunningMR - mitral regurgitationERO - Effective Regurgitant Orifice (cm2)PM - papillary musclesIVS - interventricular septumLW - lateral wallAW - anterior wallIW- inferior wallDSE - dobutamine stress echocardiography LDD - low-dose dobutamineWMAI – wall motion abnormality index CAS - Clinical Assessment ScaleFC- functional classPET-positron emission tomographyPCI- primary coronary interventionTLT- thrombolytic therapy
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML