-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(5): 455-462
doi:10.5923/j.ajmms.20221205.02
Received: April 12, 2022; Accepted: May 3, 2022; Published: May 10, 2022

Relationship between Chronic Kidney Disease and Oral Health
Rizaev Jasur Alimjanovoch, Khusanbaeva Feruza Akmalovna, Khazratov Alisher Isamiddinovich
Samarkand State Medical Institute, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article describes the prevalence and intensity of dental diseases in patients with CKD in Uzbekistan. The results obtained after the use of standard dental treatment are presented: data on the intensity and prevalence of caries, the dynamics of the hygienic state, periodontal status and the state of the oral mucosa, assessment of the fillings state in patients with CKD.
Keywords: Caries, Prevalence, Intensity, Non-carious lesions, Periodontal disease, Oral mucosa, Dentistry, Chronic kidney disease, CKD
Cite this paper: Rizaev Jasur Alimjanovoch, Khusanbaeva Feruza Akmalovna, Khazratov Alisher Isamiddinovich, Relationship between Chronic Kidney Disease and Oral Health, American Journal of Medicine and Medical Sciences, Vol. 12 No. 5, 2022, pp. 455-462. doi: 10.5923/j.ajmms.20221205.02.
Article Outline
1. Introduction
- Almost all chronic diseases of the body are associated with poor oral health, which leads to the need for better dental care. This is especially evident in patients with chronic kidney disease, where oral disease is a potential cause of deterioration in their already fragile health. [18,19].Existing data on the prevalence and severity of oral disease in patients with chronic kidney disease are limited to small samples. Studies show various cases of oral diseases in such patients. However, based on this meager data, it is estimated that almost 90% of patients with chronic kidney disease exhibit some oral disease symptoms, especially gingival hyperplasia, xerostomia, and changes in salivation and salivary composition [4].Patients with chronic kidney disease require special attention from the dentist due to multiple manifestations in the oral cavity, side effects and treatment of the underlying disease [4].The need to study the manifestation of CKD in the oral cavity in patients in Uzbekistan was the purpose of this study.
2. Study Materials and Methods
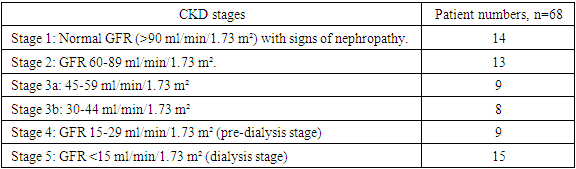
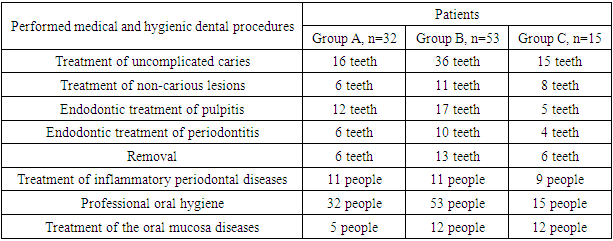
- The study involved 100 people, including 68 patients with CKD, of which 15 patients received hemodialysis. 32 practically healthy people made up the control group. The age of the patients was 45-56 years. Men - 58, women - 42 people. The study took place in 2020-2022. on the basis of the Samarkand regional multidisciplinary center and third clinic of the Tashkent Medical Academy.The patients were divided into the following groups:1. A group of persons without pathology of the urinary system - 32 people (group A);2. Patients with chronic kidney disease who are not on hemodialysis treatment - 53 people (group B);3. Patients with chronic kidney disease undergoing hemodialysis treatment - 15 people (group B).
|
|
3. Dental Status Study Results
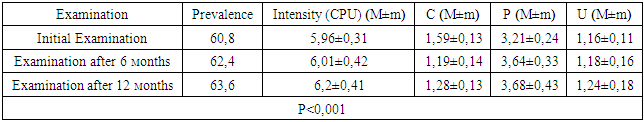
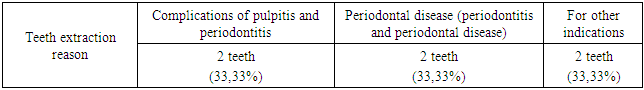
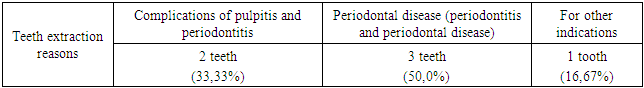
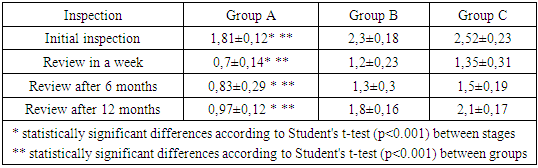
- Group A patients were treated for carious teeth, non-carious lesions, endodontic treatment and extraction of non-treatable teeth. In total, 22 teeth were treated for carious and non-carious lesions in this group, 18 teeth were treated endodontically for pulpitis and periodontitis, and 6 teeth were removed (the main reason was complications of pulpitis and periodontitis - 33.33%). At the initial examination, the number of filled teeth was 3.21±0.24, when examined after 6 months, the number of filled teeth increased to 3.64±0.33, after 12 months - 3.68±0.43. The number of teeth affected by the carious process decreased from 1.59±0.13 at the first examination to 1.28±0.13 at the examination a year later (reduction by 24%). The increase in the prevalence of caries for 1 year is 2.8%.In this group, the "P" component is predominant: 3.21±0.24-3.68±0.43, against "C" - 1.19±0.14-1.59±0.13 and "U" - 1.16±0.11-1.24±0.18. We attribute this to the medical and hygienic measures taken.Assessment of the state of seals after six months revealed their safety in 98.1% of cases, after a year - in 95.7%.The radiological method revealed the absence of periapical lesions in endodontically treated teeth in 97.3% after 6 months and 95.2% after 12 months.
|
|
|
|
|
|
|
|
|
|
|
4. Study of Dental Status Discussion Results
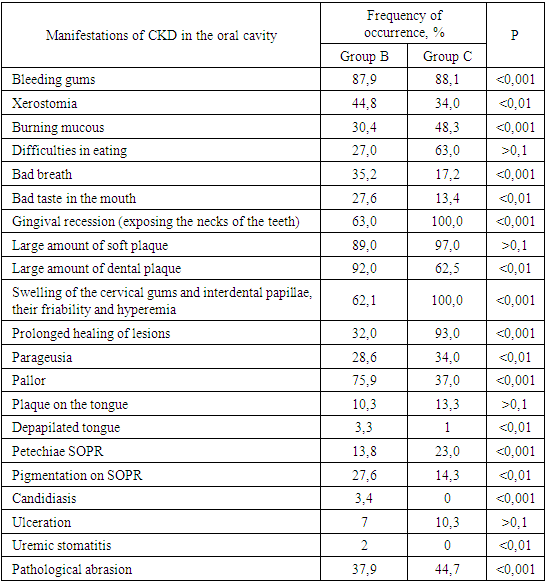
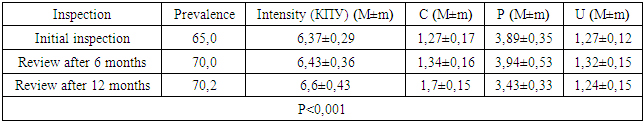
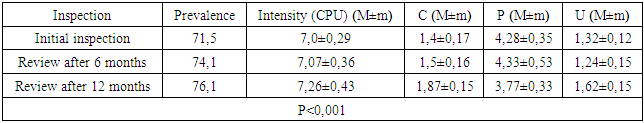
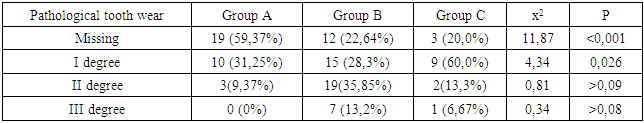
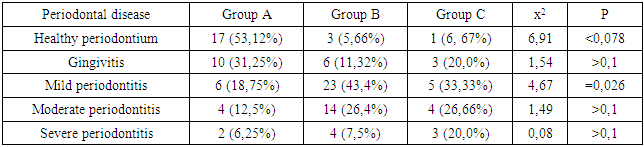
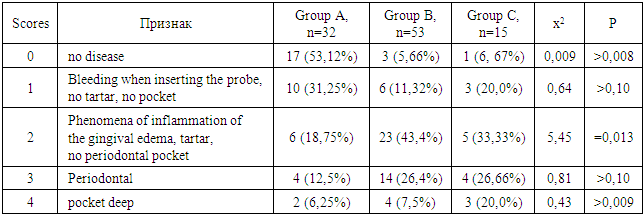
- We found that in all groups of patients with CKD, the prevalence and intensity of caries were higher than in individuals without urological diseases.Our data indicate that all patients with CKD have an unsatisfactory hygienic level of the oral cavity, which coincides with the data of studies by many authors.As a result, after the preventive measures taken a year later, the prevalence of caries in group A was 63.6% (an increase of 2.8% compared with the initial examination), the intensity of caries changed this period of time from 5.96 ± 0.31 to 6.2 ±0.41 (p<0.01), the increase in caries was 4.02%;. Examination after 12 months revealed the number of carious teeth equal to an average of 1.28±0.13.When examined after a year of patients in group B, the increase in the prevalence of caries was 5.2%, with an intensity of 6.6 ± 0.43, the increase in the intensity of caries was 3.6%.In group B, the increase in the prevalence of caries after 12 months was 5.4%. The intensity was 7.26±0.43, the increase in the intensity of caries was 3.71%.Minor changes in the prevalence and intensity of dental caries clearly demonstrate that in patients with CKD these indicators are greatly affected by an increased level of urea in saliva, even despite a decrease in the mineralizing properties of the oral fluid and a deterioration in oral hygiene.So, if during the examination a week after cleaning in group B the hygienic index was detected at the level of 1.2 ± 0.23 (during the initial examination, before cleaning - 2.3 ± 0.18), then after 12 months it was 1.8 ±0.16 (increase by 50%).We can observe similar results in group B. During the initial examination, the hygienic index was 2.52±0.23, a week after cleaning - 1.35±0.35, and a year later it increased to 2.1±0.17 (increase by 55.55%).While in group A, the hygiene index increased by 38.5% - from 0.7±0.14 a week after cleaning to 0.97±0.12 a year later.Deterioration of the hygienic state in groups with CKD after a year is the result of a deterioration in the processes of natural self-purification, a decrease in the activity of local immunity. In this regard, we believe that doctors should motivate patients to perform individual hygiene procedures.Abnormal wear of hard dental tissues (about 80% of patients with CKD) may be associated with uremia, which usually occurs in patients with CKD. It has been suggested that hypocalcemia secondary to chronic kidney disease, which contributes to renal osteodystrophy, is one of the causes of non-carious lesions of dental hard tissues [25].Hyperpigmentation (27.6% and 14.3% in groups B and C) is likely to be associated with deficiency of beta-melanocyte-stimulating hormone secreted by the kidneys. As a result, excess melanin is deposited in the basal layer of the oral epithelium [23].Swelling and pastosity of the gums (62.1 and 100% in groups B and C, respectively) can be caused by drugs taken by patients, which can be divided into three main groups: anticonvulsants, immunosuppressants, and calcium channel blockers. However, the exact cause of drug-induced gingival hyperplasia is unknown; however, the condition is believed to be associated with some risk factors that contribute to gingival inflammation, such as poor oral hygiene, presence of plaque, dose and duration of the drug used [24].High levels of urea (16.34±0.88 and 27.24±0.83 mmol/l in groups B and C), dimethyl- and trimethylamines, and low levels of zinc may be associated with reduced taste perception in patients with uremia [25]. An increased concentration of urea, which is cleaved by salivary urease into ammonia and carbon dioxide, gives a sensation of a metallic, unpleasant aftertaste [25]. The mechanisms underlying the changes in taste perception in patients with uremia are unknown, but they are probably related to the influence of uremic toxins on the central nervous system and peripheral nervous system (taste buds) [14]. Bleeding gums were observed in 87.9% and 88.1% of patients with CKD and were associated with poor oral hygiene and periodontal inflammation [15].Burning sensation in the mouth, which was significantly higher in patients with CKD (30.4% in group B and 48.3% in group C), was associated with dry mouth, peripheral nerve damage from urinary toxins, and drug effects [3,16].Complaints of xerostomia in patients with CKD (44.8 and 34.0% in groups B and C, respectively) are associated with fluid restriction, electrolyte imbalance, use of certain drugs such as furosemide and hydrochlorothiazide (antihypertensive drugs), mouth breathing, alteration of the glands (atrophy of the parenchyma of the small salivary glands), leading to a decrease in the secretion of saliva [20].Halitosis was reported by 35.2% and 17.2% of participants in this study (groups B and C, respectively). The uremic malodor or halitosis reported by patients with CKD is an ammoniacal odor that is caused by a high concentration of urea in saliva and is broken down to ammonia [22]. This is due to the reduced function of the kidneys to remove urea from the body, therefore, the concentration of urea in the blood (uremia) increases, as well as in saliva. In addition, patients with CKD often neglected oral hygiene [20].Pallor of the OM (75.9 and 37.0% in groups B and C), petechiae (13.8 and 23.0%) may be associated with anemia, be the result of anticoagulant therapy and/or platelet dysfunction [26].The prevalence of periodontitis (31.2 and 47.0% in groups B and C) in this patient population is increasing, most likely due to immunosuppression in uremia. This suppresses the inflammatory reaction of the gums during the accumulation of plaque [1]. Periodontitis is associated with elevated values of other components of the acute phase of inflammation, including lower concentrations of high-density lipoproteins [12,13], elevated levels of low-density lipoproteins [14-16] and neutrophils [17]. Inflammatory lesions of the periodontium in patients with CKD occur against the background of dystrophic phenomena, which are significantly more common in patients receiving hemodialysis (p=0.027). Signs of periodontal disease are found in 37% of cases in patients with CKD and in 56% of cases in patients of group B.Deterioration of the hygienic status of the oral cavity affects the severity of inflammation (p=0.004). Elevated blood creatinine leads to the development of osteodystrophic phenomena and dystrophy in periodontal tissues. (p=0.03).Chronic kidney disease and periodontal disease are also associated with common risk factors such as diabetes mellitus [2], age and tobacco smoking [6]. Periodontal disease is thought to be a non-traditional risk factor for chronic kidney disease due to systemic changes caused by periodontal inflammation. Due to the periodontogenic presence of bacteria, inflammatory mediators such as interleukin-1, interleukin-6, prostaglandin 2, and tumor necrosis factor-alpha are locally produced, and their antigens can enter the bloodstream [9]. Studies have shown that compared with healthy people, patients with periodontitis may have elevated levels of C-reactive protein and, as a result, a mild systemic acute-phase inflammatory reaction. There seems to be a mutual influence between periodontopathies and chronic kidney disease. Because affected periodontal tissue is susceptible to chronic inflammation, it is likely that oral bacteria may influence the course of chronic kidney disease. In patients with chronic kidney disease, a greater number of periodontal red complex bacteria (P. gingivalis, T. forsythia, T. denticola) and C. albicans, as well as significant destruction of periodontal tissue [18], as well as a greater number of periodontal bacteria (P. gingivalis, T. forsythia, P. intermedia and P. nigrescens, A. actonomycemconcomitans) in periodontal pockets in these patients [19]. Therefore, regular assessment and prevention of periodontitis are of particular importance for patients with chronic kidney disease.The lesions detected in uremic stomatitis (2% in group B) were very painful and were most often localized on the lower surface of the tongue (81%). It manifested itself in the form of a gray pseudomembrane covering painful spots of erythema or red ulcers with a "purulent" cover.Studies on the condition of the periodontium in patients with CKD indicate poor oral hygiene (in patients with CKD of both groups, a large amount of soft and hard plaque was found) and gingivitis [4,6-10]. This is probably a consequence of a pronounced uremic syndrome associated with impaired immune function, as well as altered activity of lymphocytes and monocytes [11].The results obtained give us reason to assert that there is a need to develop a scheme for more effective treatment of dental diseases in patients with CKD, for which our study will continue.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML