-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(4): 373-378
doi:10.5923/j.ajmms.20221204.02
Received: Mar. 1, 2022; Accepted: Mar. 18, 2022; Published: Apr. 15, 2022

Features of the Course of Chronic Hepatitis “B” in Children Depending on the Carrier of the Gene Phenotype
Inoyatova Flora Ilyasovna, Kadyrkhodzhayeva Khilola Marufovna, Inogamova Gulnoza Zakhidjanovna, Ikramova Nodira Anvarovna, Abdullayeva Feruza Gafurovna, Valiyeva Nargiza Kabildzhanovna, Akhmedova Akida Khotamovna
Republican Specialized Scientific and Practical Medical Center of Pediatrics of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

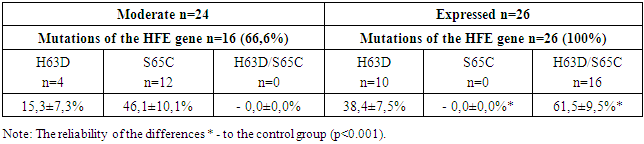
Objective: Purpose: to establish the frequency and clinical significance of mutant variations of the HFE gene polymorphism in chronic hepatitis B (CHB) in children with iron overload syndrome (IOS). Materials and methods: 60 children with chronic hepatitis B with iron overload syndrome (IOS) were examined. When distributing children into groups, we took into account the criteria we developed for assessing the degree of life expectancy in children with CHB: CST>0.5 - mild degree of life expectancy (43.3% of children), CST <0.5 - moderate severity of life expectancy (31.7% of children) and CST <0.2 - severe degree of SPL (25.0%). Virological verification of HBV was performed by ELISA and PCR. Using PCR Real Time and molecular genetic analysis, HFE gene C282Y, H63D, S65C mutations were detected from amplified DNA using the PRONTO Hemochromatosis reagent kit (Israel). The transferrin saturation coefficient (CST) was calculated using the formula CST = sTfR / log10. Ft. Results: Results: The study of the hemochromatosis gene HFE showed that the overwhelming majority (84.0%) of children with CHB with IOS were carriers of heterozygous, phenotypically different, mutant types. And only 16.0% of sick children were homozites of the wild (normal) HFE gene. Analysis of the phenotypic polymorphism of the hemochromatosis gene HFE revealed the presence of three point heterozygous mutations: H63D, S65C and combined variations in H63D / S65C, the latter of which is associated with severe forms of CHB and severe IOS. Conclusion. Children with CHB with IOS are characterized by a high incidence of heterozygous mutations in the HFE gene, the phenotypic manifestations of which were S65C, H63D, H63D / S65C. The comparability of the heterozygous combined mutation H63D / S65C with severe forms of CHB and a severe degree of IOS gives grounds to consider this phenotype of the HFE gene as a factor in the progression of the disease.
Keywords: Children, Chronic hepatitis B, Iron overload syndrome, HFE hemahromatosis gene
Cite this paper: Inoyatova Flora Ilyasovna, Kadyrkhodzhayeva Khilola Marufovna, Inogamova Gulnoza Zakhidjanovna, Ikramova Nodira Anvarovna, Abdullayeva Feruza Gafurovna, Valiyeva Nargiza Kabildzhanovna, Akhmedova Akida Khotamovna, Features of the Course of Chronic Hepatitis “B” in Children Depending on the Carrier of the Gene Phenotype, American Journal of Medicine and Medical Sciences, Vol. 12 No. 4, 2022, pp. 373-378. doi: 10.5923/j.ajmms.20221204.02.
Article Outline
1. Introduction
- Among the multifactorial causes (social and biomedical) of the progression of liver damage, it is the viral etiology that is often complicated by various pathological conditions that occur in the perspective of concomitant diseases, cause the development of two or more mutually reinforcing processes. The fact of the association of chronic hepatitis B (CHB) and anemia of inflammation (up to 78% among other anemias) is beyond doubt, especially in cases when the process is accompanied by iron overload syndrome (IOS), considered as a promoter of necro-inflammatory activity, fibrosis and progression of the pathological process in the liver [1,2,3,4,5,31]. In 40-65% of cases, anemia of inflammation is expressed as refractory to ferrotherapy, the determining factor of which is iron overload of the body [6,7,8,9,10]. In our studies, a high incidence of anemia of inflammation was established, where the percentage representation corresponded to 95.6%, of which in 60.7% of cases the course of anemia was designated as refractory (tolerance to ferro therapy), the cause of which was iron overload of the body. It is important to note that the IOS in children is formed slowly and depends on the duration of the disease. Therefore, in patients with liver pathology, it is impossible to be absolutely sure that there is no excessive accumulation of iron even when transferrin is saturated with iron <45% [11,12,13,14,30]. Considering from the standpoint of the development of IOS, the genetic factor HFE-transmembrane protein, which belongs to the family of the main histocompatibility complex of class 1, responsible for limiting iron absorption in the intestine, is of great importance [15,16,17,29]. The priority of the HFE gene in iron homeostasis is confirmed by a mutation of this protein (C282U, H63D and S65C), which leads to severe iron overload of the body and hemochromatosis due to the unlimited interaction of transferrin with its receptor and the constant accumulation of iron in tissues [18,19,20,21]. The presence of a combination of factors in patients with liver pathology: violation of synthetic liver function, carriage of minor (heterozygous) mutations of the HFE gene allows us to assess the risk of secondary IOS formation as high [21,22,23]. In evidence of this, the results of other scientists showed that in patients with rapid progression of liver fibrosis, the frequency of mutation of the HFE gene reached up to 64.2% [24,25]. At the same time, this problem has not been studied in children with CHB, where the results of mutation of the HFE gene may manifest themselves ambiguously in different conditions and populations, which requires study.
2. Aim of the Study
- In this regard, the aim of the study was to establish the frequency and clinical significance of mutant variations of the HFE gene polymorphism in chronic hepatitis B in children with iron overload syndrome.
3. Materials and Methods
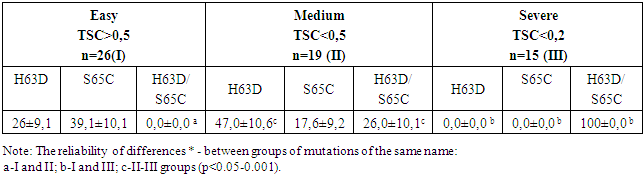
- 60 children with chronic hepatitis B with iron overload syndrome aged from 4 to 18 years were examined. Of these, 76.8% are boys, 23.2% are girls. The diagnosis of CHB was established on the basis of anamnestic, clinical, laboratory and instrumental studies in accordance with the criteria for diagnosing the degree of activity of the pathological process in the liver in children [26,30]. When diagnosing iron overload syndrome, the Algorithm for the Differential Diagnosis of Inflammatory Anemia in Children with CHB was also used [27,31]. The transferrin saturation coefficient (TSC) was calculated using the formula TSC= sTfR/log10. Ft. When distributing children into groups, the developed criteria for assessing the degree of IOS in children with CHB were taken into account: TSC>0.5 - mild degree of IOS (43.3% of children), TSC<0.5 - moderate degree of IOS (31.7% of children) and TSC<0.2 - severe degree of IOS (25.0%). The control group consisted of 30 practically healthy children.Virological verification was carried out based on the detection of HBsAg, HBsAb, HBeAg, HBeAb, HCVAb, HDVAb by the ELISA method using "HUMAN" kits (Germany) on the "MULTISKAN FC" device. A blood test for the detection of HBV-DNA was performed by PCR with hybridization-fluorescence detection in "real time" mode on the BIO-RAD iQ5 amplifier (USA) using the sets "HBV-FL, HCV-FL, HDV-FL AmpliCensR" (Russia) in the clinical and experimental laboratory of the RSSPMC Pediatrics. The ELISA method was used to determine: sTfR (soluble transferrin receptors), FR (ferritin) in blood serum using Accu Bing ELISA Microwells (USA) kits. The transferrin saturation coefficient (TSC) was calculated using the formula sTfR/Log ferritin. Real-Time PCR and molecular genetic analysis were used to detect mutations of C282Y, H63D, S65C of the HFE gene from amplified DNA using a set of reagents "PRONTO Hemochromatosis" (Israel). Statistical data processing was carried out by the method of variational statistics using an Excel program and calculating the Student's t-test.
4. Results and Discussion
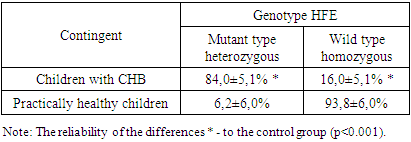
- The molecular genetic analysis made it possible to exclude the hereditary nature and confirm the secondary nature of IOS in children with CHB. No homozygous point mutations of the C282Y HFE gene have been identified in any case, where C282Y leads to the replacement of cysteine with tyrosine, making it impossible to form a disulfide bond by altering protein folding [12,13]. The study of the HFE gene showed (Table 1) that the majority (84.0%) of children with CHB with IOS were carriers of heterozygous, phenotypically different, mutant types (p<0.001 to control).
|
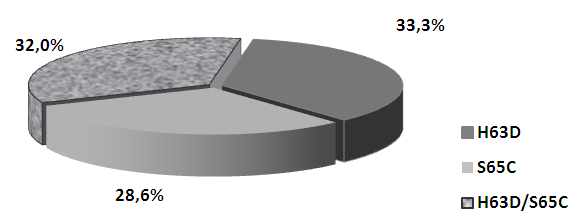 | Figure 1. Phenotypic polymorphism of the HFE gene in children with CHB with IOS |
|
|
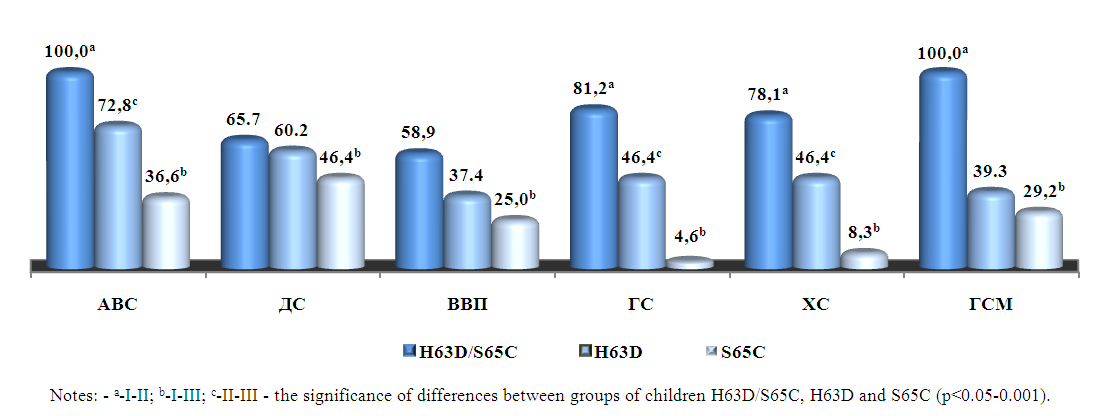 | Figure 2. Clinical syndromes of CHB depending on the degree of IOS in children |
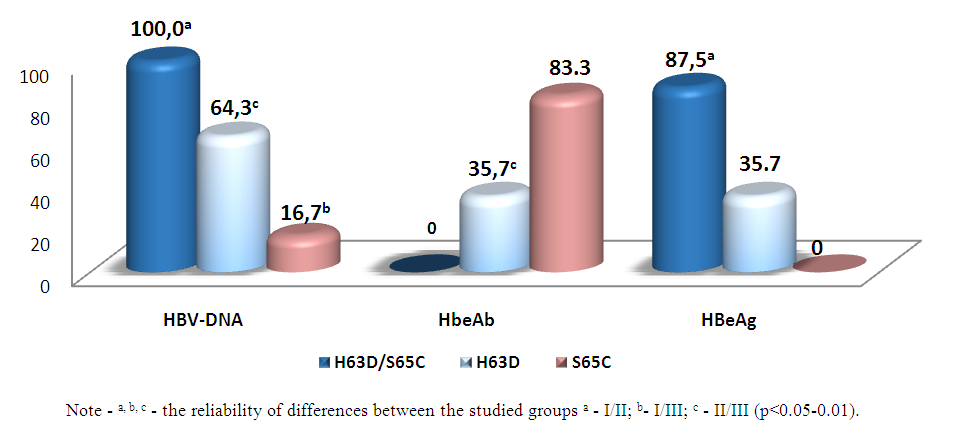 | Figure 3. Marker profile of CHB in children with IOS depending on HFE phenotypes, % |
5. Conclusions
- 1. For children with CHB with IOS, the analysis of the phenotypic polymorphism of the HFE gene revealed the presence of three point heterozygous mutations: H63D, S65C and combined variations of H63D/S65C. The comparability of heterozygous combined H63D/S65C mutations of the HFE gene with pronounced forms of CHB and severe IOS gives reason to consider this phenotype of the HFE gene as a factor of disease progression.2. In children with CHB with IOS, the replicative phase of HBV viral infection depends on the carrier of the HFE phenotype, where the most pronounced viral aggression is observed with a combined H63D/S65C mutation with a high viral load of HBV-DNA and high persistence of HBeAg.3. The features of the clinical course of CHB with IOS in children with a complex H63D/S65C mutation of the HFE gene are the prevalence of pronounced and progressive forms of the disease with a persistent predominance of syndromes: asthenovegetative, hemorrhagic, cholestatic and pronounced hepatosplenomegaly. Such conditionally specific symptoms as orthostatic vertigo and epithelial syndrome are informative in the diagnosis of IOS in children with CHB. At the same time, the leading biochemical indicators of liver dysfunction in children with a complex H63D/S65C mutation were such syndromes as cytolytic with the development of prolonged hyperfermentemia, cholestatic and mesenchymal inflammatory.Information about the source of support in the form of grants, equipment, and drugs. The authors did not receive financial support from manufacturers of medicines and medical equipment.Conflicts of interest: The authors have no conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML