-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(3): 361-364
doi:10.5923/j.ajmms.20221203.28
Received: Mar. 7, 2022; Accepted: Mar. 21, 2022; Published: Mar. 28, 2022

Role of Polymorphisms Pro72Arg in the Gene TP53 and CALR in the Diagnosis of Essential Thrombocytemia
Musashaykhova Shakhnoza Mamirbekovna1, Musashaykhov Khusanboy Tadjibayevich1, Boboev Kodirjon Tukhtabaevich2
1Andijan State Medical Institute, Andijan, Uzbekistan
2Republican Specialized Scientific and Practical Medical Center of Hematology of the Ministry of Health of the Republic of Uzbekistan
Correspondence to: Musashaykhova Shakhnoza Mamirbekovna, Andijan State Medical Institute, Andijan, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the course of the study in 54 patients with essential thrombocytemia, the frequency of occurrence of the genetic markers Pro/Arg of the TP53 gene and CALR in the formation of ET was analyzed. The comparison group consisted of 48 patients with PMF. In the studied groups, the actual distribution of the Pro/Arg genotypes of the TP53 and CALR gene corresponded to what was expected at the Hardy-Weinberg equilibrium (HWE) (p<0.05).
Keywords: ET, PMF, Polymorphic gene variants, Tumor growth suppressor, Pro72Arg gen TP53, Polymorphism
Cite this paper: Musashaykhova Shakhnoza Mamirbekovna, Musashaykhov Khusanboy Tadjibayevich, Boboev Kodirjon Tukhtabaevich, Role of Polymorphisms Pro72Arg in the Gene TP53 and CALR in the Diagnosis of Essential Thrombocytemia, American Journal of Medicine and Medical Sciences, Vol. 12 No. 3, 2022, pp. 361-364. doi: 10.5923/j.ajmms.20221203.28.
Article Outline
1. Introduction
- Essential thrombocytemia (ET) refers to Ph-negative myeloproliferative diseases (MPD) and is a pathology of clonal hematopoietic stem cells, which leads to increased platelet formation. The leading hypothesis is the polyetiological nature of the occurrence of the disease, where the predisposition to the disease is realized under the influence of external factors that damage the genome of a normal cell and lead to its malignant transformation. Assessment of the mutational status of JAK2, MPL and CALR is important not only for diagnosis, but also for the prognosis of both thrombotic complications and overall survival. Patients with three mutations (TN status) remain the least studied category. The prognosis in patients with TN is currently recognized as unfavorable [1].Thus, the molecular genetic study of mutations JAK2V617F, JAK2 exon12, MPLW515K/L and CALR plays an exceptional role in the diagnosis of classical Ph-negative MPD. Nevertheless, genes controlling the transmission of signals inside the cell, chromatin remodeling, DNA methylation, oncogenes and tumor suppressors are involved in the occurrence and development of these diseases [2]. In addition to three somatic mutations (JAK2V617F, MPL and CALR) activating the JAK-STAT pathway, a spectrum of various epigenetic rearrangements was revealed during ET: TET2, EZH2, ASXL1, CBL, IDH, IKZF1, LNK, IDH1/IDH2. Somatic mutations of the TET2, EZH2, DNMT3A and ASXL1 genes play an essential role in the pathogenesis of MPD, determine the phenotype and prognosis of the disease. Somatic mutations can occur before the appearance of a clone with the JAK2 mutation, simultaneously or as a later molecular event during the progression of the disease [3]. The lack of convincing data in favor of the specificity of such mutations for ET, their detection in other diseases of the blood system, organs of lymphoid and hematopoietic tissues does not allow us to talk about the pathogenetic role of these rearrangements and their inclusion in the diagnostic algorithm of ET as highly specific molecular genetic markers of clonality. Nevertheless, the contribution of epigenetic mutations to the occurrence and development of ET requires further study.The TP53 gene encodes a tumor suppressor protein involved in regulating the expression of target genes regulating the cell cycle, apoptosis, and DNA repair. The loss of gene function is associated with the appearance of various malignant human tumors. The mutation of the TP53 gene was detected in 45.5% of MPD in the blast crisis phase and only in 4% in the chronic phase [4]. Thus, TP53 mutations play an important role in the process of disease transformation. Nevertheless, despite modern advances in understanding the etiology and pathogenesis of the disease, there is currently no empirically confirmed, unified and generally accepted concept of the pathogenesis of ET. The leading hypothesis of the pathogenesis of the disease is polyethological. The predisposition to the development of ET, as well as other diseases of the Ph’-negative MPD group, is realized when a hematopoietic stem cell is exposed to various external factors that damage its genome with subsequent malignant transformation of the cell [5]. Additional studies are needed to clarify the role of other molecular events in the formation of the phenotype of each individual nosology in the Ph-negative MPD group. The new data are of indisputable importance for the synthesis of targeted drugs. Adequate diagnosis and regular monitoring of treatment with the help of clinical, morphological, cytogenetic and molecular genetic research methods is a prerequisite for the correct prediction of the course of the disease and achieving maximum effectiveness of therapy. Currently, there are no generally accepted standards for the diagnosis and treatment of ET in domestic clinical practice. Thus, the study of ET should be continued in order to identify and describe new specific molecular genetic markers of clonality, which may contribute to a deeper understanding of the nature of this malignant myeloproliferative disease. It seems appropriate to study the issue of the influence of genetic rearrangements on the features of the clinical course, the possible potentiation of the risks of complications and, in general, the prognosis of ET. Only a detailed study of the population features of the above-mentioned genetic mutations and an assessment of the correlation between the genotype and phenotype of the disease can make it possible to choose the right strategy for early diagnosis and prediction, development of preventive measures for thromboembolic and hemorrhagic complications in patients with ET. Finally, there are many unresolved questions regarding the expediency of diagnosing certain gene polymorphisms in clinical practice, which is largely explained by the insufficient number of studies aimed at establishing correlative links between the features of the clinical course of ET and the presence of certain markers in the patient's genotype. The question of which interactions of acquired and genetic factors, as well as gene-gene combinations, determine the predisposition to the development of thrombosis and hemorrhage, the features of the course remains open. The need to use fundamentally new approaches in studying the fundamentals of genetic predisposition to such types of complications in ET is dictated by the current concept of the polygenic nature of myeloproliferative diseases, which postulates the presence in the vast majority of cases of thromboembolic diseases of not one, but several genetic variants that independently or synergistically modify the risk of developing the disease.The obtained data will improve the assessment of the clinical and prognostic significance of the carrier of molecular genetic rearrangements in ET and will contribute to the updating of therapeutic approaches and algorithms, which will optimize the treatment and personalize the tactics of therapy for this disease.
2. Main Body
2.1. Purpose of the Study
- Analysis of the contribution of the genetic markers Pro72Arg in the TP53 and CALR gene to the development of ET by comparing a group of patients with PMF.
2.2. Material and Methods of Research
- To solve the tasks set, we conducted a genetic study in 54 patients with ET who were treated at the Republican Specialized Scientific and Practical Medical Center of Hematology of the Ministry of Health of the Republic of Uzbekistan (RSSPMCG of the Ministry of Health of the Republic of Uzbekistan), who made up the main group. The comparison group consisted of 48 patients with PMF. Molecular genetic studies were carried out in the Department of Molecular Medicine and Cellular Technologies of the RSSPMCG of Hematology of the Republic of Uzbekistan. The diagnosis of PMF was carried out in accordance with the currently accepted recommendations and was established on the basis of the clinical picture, clinical and laboratory data.Genotyping of polymorphisms Pro72Arg in the gene TP53 and CALR it was carried out on the basis of the method of Tag Man probes on an amplifier Rotor-Gene Q (Quagen Germany), using the commercial test kit of Syntol LLC (Russia).Statistical processing of the results was performed using a standard application software package OpenEpi V.9.2. Analysis of the deviation of empirical genotype frequencies from The theoretically expected Hardy-Weinberg distribution was performed using the Statistica 6.0 software package.
2.3. The Results Obtained and Their Discussion
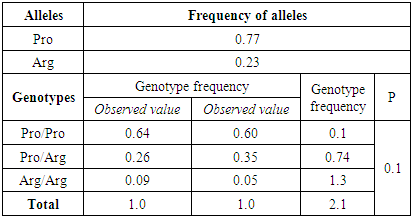
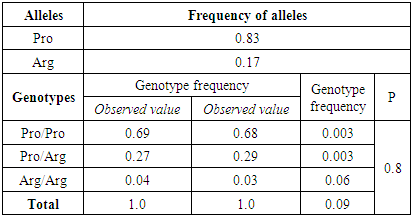
- Molecular genetic study of mutations in the genes CALRinsTTGTC, CALR52del plays an exceptional role in the diagnosis of classical Ph-negative MPD. Nevertheless, genes controlling the transmission of signals inside the cell, chromatin remodeling, DNA methylation, oncogenes and tumor suppressors are involved in the occurrence and development of these diseases. The calculation results showed that in the studied groups, the actual distribution of the Pro72Arg polymorphism genotypes in the TP53 gene did not deviate from the expected ones and corresponded to the canonical Hardy-Weinberg equilibrium (HWE) (p<0.05). The frequency of Pro and Arg alleles in the group of patients with ET was 0.77/0.23, respectively, and in the group of patients with PMF 0.83/0.17 (Tables 1, 2).
|
|
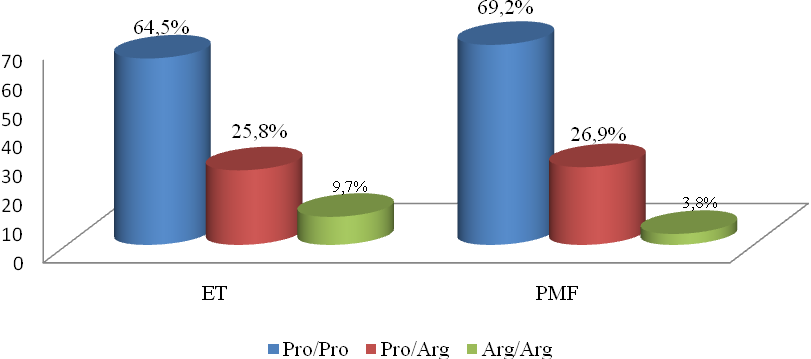 | Figure 1. Distribution of Pro72Arg polymorphism genotypes in TP53 gene in a group of patients with ET (n=31) and PMF (n=26) |
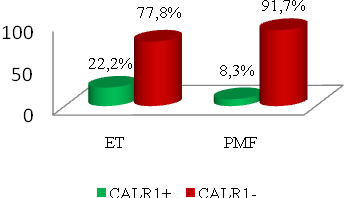 | Figure 2. Distribution of CALR1 polymorphism genotypes (insTTGTC) in the groups of patients with ET (n=54) and in the PMF group (n=48) |
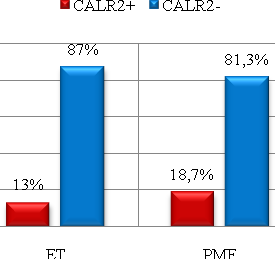 | Figure 3. Distribution of CALR2 polymorphism genotypes (CALR52del) in the groups of patients with ET (n=54) and in the PMF group (n=48) |
3. Conclusions
- Thus, the chance of detecting ET increased in the presence of an unfavorable Arg/Arg genotype of the TP53 gene by 2.7 times and with the carrier of the CALR1 positive genotype (insTTGTC) by 3.1 times in the group of patients with ET compared to the group of patients with PMF. Based on the results obtained, it can be concluded that unfavorable genotypic variants of TP53 and CALR1 positive genotype polymorphism (insTTGTC) can play a significant independent role in the development of ET, which may be an additional criterion for the diagnosis of ET and substantiation of pathogenetic approaches in carrying out therapeutic and preventive measures.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML