M. A. Abdullayeva
Bukhara State Medical Institute, Uzbekistan
Correspondence to: M. A. Abdullayeva, Bukhara State Medical Institute, Uzbekistan.
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article is devoted to the identification of morphological and morphometric changes in the aortic wall after treatment of experimental radiation sickness with ASD. Gamma-ray disease is provoked by the introduction of 3, 6, 9, 12 months of gamma radiation to rabbits at a dose of 1 Gray for 10 days. Materials and methods. During the treatment with ASD-2 radiation, there is a loss of dystrophy, destruction and inflammatory processes that develop in the aortic wall, it was determined that only a small degree of edema and dystrophic changes in the intimate part remained in the first period of the experiment. The results. In recent periods of experience, as a result of the treatment of ASD, there is a predominance of intimate regeneration and proliferation processes, hypertrophy of smooth muscle cells in the environment, proliferation of focal lymphohistiocytic cells among them.
Keywords:
ASD-2, Aorta, Endothelium, Intima, Mediastinum, Adventitia, Morphology, Morphometry
Cite this paper: M. A. Abdullayeva, Morphological and Morphometric Changes in the Aorta after Treatment of ASD-2 Experimental Radiation Sickness, American Journal of Medicine and Medical Sciences, Vol. 12 No. 3, 2022, pp. 309-313. doi: 10.5923/j.ajmms.20221203.16.
1. Introduction
The antiseptic stimulant Dorogov (ASD-2), which develops fungal products, is widely used (Kotik A.N. et al. 2002). Most mycotoxicants have teratogenic, mutogenic, allergenic, and immunodepressive properties. Treatment of injuries combined with radiation and mycotoxicants has not been developed. Therefore, the study of the mechanism of action of gamma radiation and biostimulatory ASD-2 and the morphological changes that develop in the body of animals is an important issue in all areas of veterinary medicine, even. Changes in the anatomical, topographic, and histological structures of the aorta are increasing in various scientific studies of changes in the organs of the cardiovascular system under the influence of various exogenous and endogenous factors, including radiation [1–3]. According to the authors, under the influence of the above factors, the organometric parameters, biomechanical properties of the aorta change. As a result of these effects, the wall of the aorta thickens, the lining changes, the diameter expands, and the ratio of the layers of the aortic wall to each other changes to varying degrees [4–6]. Not only under the influence of internal and external factors, but also as a person gets older, changes occur in the layers of the aortic wall due to the process of physiosclerosis. Under the influence of additional exogenous factors to the processes of atrophy that develop with age, a person begins to develop specific changes in the intima of the aortic wall. In most cases, pathomorphological changes develop from metabolism, including the absorption of cholesterol and lipids Because each of the layers of the aortic wall has a unique tissue structure, more dystrophic and destructive intima may develop, basal membrane mucoid and fibrinoid swelling, swelling in elastic fibers and fibroelastosis, hyperplasia, hypertrophy, and sometimes metaplasia and dysplasia in smooth muscle cells. Given the above discussion, modeling of pathomorphological changes in the human body, including the aorta, the most important organ of the cardiovascular system, in experimental animals and determining the degree of stabilization, reversal and cessation of light after treatment with biostimulants is an important issue [7–10].Under the influence of biostimulators, hemodynamic disturbances caused by ionizing radiation in all organs of the body, including the aortic wall, both dystrophic and metabolic changes are expected to slow down and return to normal. Under the influence of biostimulants from hemodynamic changes initially stops the paralytic expansion of capillaries, does not increase the permeability of the wall, stabilizes the endothelial layer in thick blood vessels. It has been proven that radiation initially affects the atoms and molecules of cell structures and leads to the development of both functional and organic changes. thus denaturing and degrading them, and the biostimulator stops this process as well, retaining the protein substances in the vascular wall [3–5].
2. Material and Method
As a material, the aorta of 6-, 12-, and 24-month-old rats was completely removed, i.e., the pelvis, chest, and abdomen were removed separately. The length, diameter, and wall thickness of all parts of the aorta were measured. Fragments were cut from each for histological examination. The experiment was conducted on rabbits. Rabbits aged 3, 6, 9, 12 months were given gamma radiation at a dose of 1 gray for 10 days and then corrected with an antiseptic stimulant Dorogova (ASD). The rabbits were anesthetized by beheading them in an instant. By separating the aorta, the length, diameter, and wall thickness of all its parts were measured. The aortic segments were hardened for 48 h in a neutralized solution of 10% formalin, then washed in running water for 3 h, dehydrated in increasing concentrations of alcohol, and paraffin was poured and the lumps were prepared. Histological incisions of 5-6 microns thickness from paraffin bricks were prepared on a special microtome. From the histological incisions, the paraffin material was dissolved in xylene and stained with hematoxylin and eosin dyes. Histological preparations were studied on 10, 20, 40 lenses of the Leyka microscope, and the necessary areas were photographed. Morphometric calculations were performed on micrographs taken on 40 microscope lenses, each with 6 images. In histological preparations, the external and internal diameter of the aorta were measured, the intra-aortic cavity and aortic wall area were measured in mm2, and the aortic wall, intima, medial, and adventitia thickness were measured in μm. To assess the functional status of the aorta, the Wogenworth index was calculated, i.e., the ratio of the area of the aortic wall to the area of the aortic cavity. Morphometric data were statistically processed in the IBM SPSS Statistics program.
3. Results and Discussion
The results of microscopic examination of the animal aorta after irradiation and its correction with antiseptic stimulator Dorogova (ASD) showed that if from the first period of the experiment (3 months) in the untreated group of animals aortic edema, dystrophy and destruction processes were observed. During the 6-month period of the experiment after the application of ASD, it was observed that there was no tumor in the intimate aortic wall, only superficial dystrophy of the fibrous structures. It was found that endothelial cells are clearly differentiated relative to the control and radiation groups, in some areas they are hypertrophied and bulge on the surface of the intima. It was found that the nuclei of these cells were mostly round in shape and stained chromatin dark. The basal membrane and elastic fibers under the endothelium are slightly thickened due to protein dystrophies such as mucoid and fibrinoid swelling, and some areas with discoloration are metachromasia (Fig. 1). In some animals in this group, the tumor process is preserved, albeit to a lesser extent, in the intima of the aortic wall, and its intima layer spreads to the connective tissue and a layer of smooth muscle cells on the surface of the media. In this case, the surface of endothelial cells is uneven, in some places the endothelial cells are swollen, in the head areas are deposited on the basement membrane. Their nuclei are of various sizes and most of them are hyperchromic chromatin. The basal membrane and elastic fibers are torn, some areas are torn, discoloration is altered, and metachromasis occurs. The connective tissue cells in the intima are relatively hypertrophied and disordered, with inflammatory cells appearing between them. 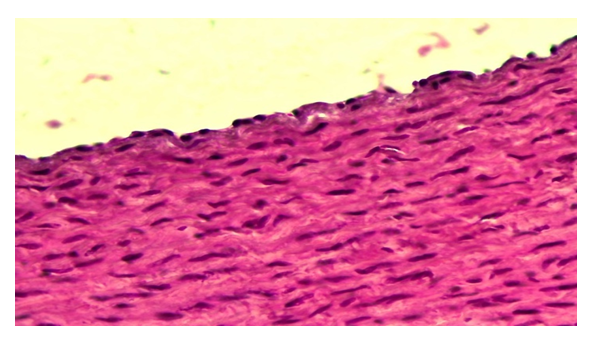 | Figure 1. Corrected group with ASD. The tumor disappears in the intimate aortic wall and the endothelium is hypertrophied. Paint: G-E. X: 10x40 |
In this group and in the first period of the experiment, the smooth muscle cells in the aortic wall media were slightly hypertrophied, disrupting the parallel placement order. The nuclei of some are enlarged and hypertrophied (Fig. 2). Myofibrils are sparse, fragmented, and have lost their tinctorial location relative to the control group. The tufts of elastic fibers between them are relatively thick and curved.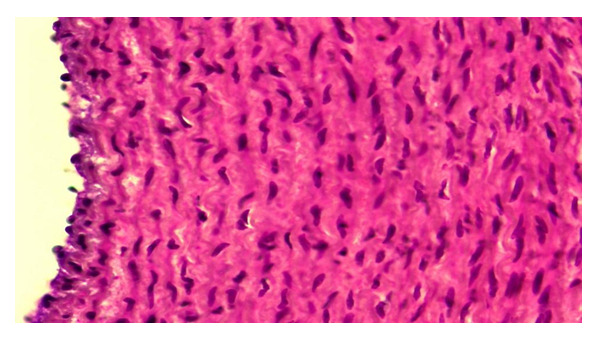 | Figure 2. Corrected group with ASD.Hypertrophy of smooth muscle cells in the aortic wall media. Paint: G-E. X: 10x40 |
Correction of changes in the aorta of animals exposed to radiation with ASD During the 12-month period of the experiment, it was observed that edema and degeneration processes in the aortic wall intima were partially preserved. However, during this period, the endothelial layer of the aortic wall is intact, only the endothelial cells become flattened and thinned, and the nuclei become smaller and flattened. It is found that the process of bitrose edema is preserved in the basal membrane below it (Fig. 3). Simultaneous swelling and protein dystrophy are also observed in the elastic fibers. As a result, it is determined that the connective tissue cells and fibers of the intima are torn and fragmented. The nuclei of connective tissue cells are found to be relatively hypertrophied and hyperchromedized, among which inflammatory cells are found.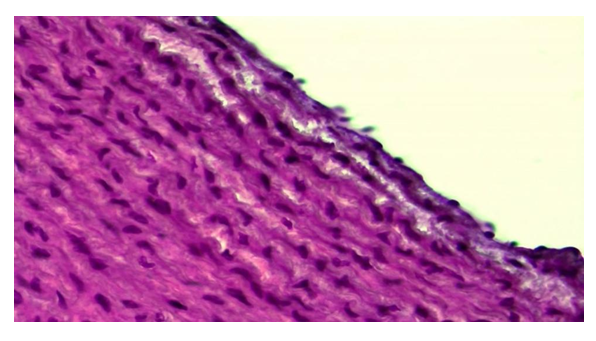 | Figure 3. Corrected group with ASD, 12-month period. Preservation of swelling and degeneration in the interstitial material of the aortic wall intimacy. Paint: G-E. X: 10x40 |
Pathomorphological changes in the aortic wall layers of animals under the influence of radiation are sharply reduced when treated with Dorogov's antiseptic stimulator. It is found that the processes of edema, dystrophy and destruction that develop, especially in the intimate aortic wall, are sharply reduced, only in some areas of the intima only the tumor of the interstitial tissue is preserved. During the 12-month period of the experiment, changes such as destruction, inflammation, necrobiosis, and calcinosis that develop under the influence of radiation were observed to disappear almost immediately after the application of ASD. During this period of the experiment, it is observed that in the intima of the aortic wall, the process of dystrophy, such as superficial mucoid swelling of the basement membrane and interstitial material, is preserved. The absence of the above-mentioned inflammatory and destructive processes in the medial layer of the aortic wall is also found, only in the interstitial tissue of the medial layer the focal tumor process is preserved (Fig. 4). In addition, it is found that the smooth layer of smooth muscle cells in the medial layer is slightly torn, the parallel placement of cells is disturbed. It is found that some of the smooth muscle cells are relatively hypertrophied, and their nuclei are hypertrophied and hyperchromatic.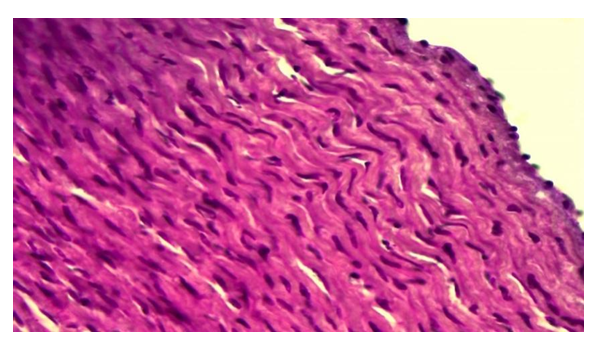 | Figure 4. Corrected group with ASD, 12-month period. Preservation of a focal tumor in the medial layer of the aortic wall. Paint: G-E. X: 10x40 |
By the 24-month period of the experiment, along with the disappearance of destructive and inflammatory processes in the radiation group, processes such as superficial edema and dystrophy, which had survived in the early stages of this series, disappeared and were replaced by regenerative and proliferative processes. If we consider the intimacy of the aortic wall, during this period of the experiment it is observed that proliferative processes predominate, i.e. hypertrophy of endothelial cells, both cytoplasm and nucleus are darkly stained and densely packed (Fig. 5). It is observed that the subendothelial basal membrane is relatively thick and darker, and in its composition the tumor process is completely lost. The connective tissue cells and fibrous structures in the intima are also thickened and hypertrophied, and their nuclei are hyperchromatic.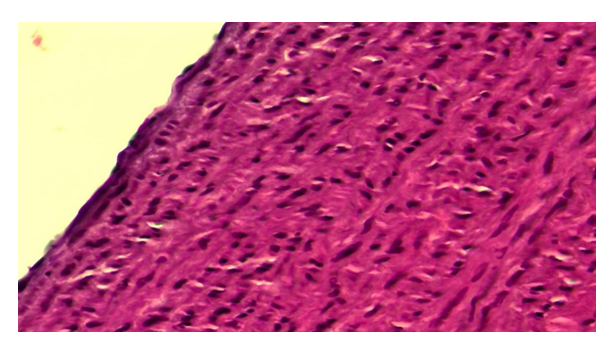 | Figure 5. Corrected group with ASD, 24-month period.Proliferation of aortic wall intimate cells. Paint: G-E. X: 10x40 |
During this period of the experiment, proliferative and inflammatory processes are observed in some areas of the aortic wall, especially in the intima. At the same time, it is determined that the endothelial cells also proliferate, become hypertrophied, and the nucleus becomes darker. It is observed that the connective tissue cells in the intima also undergo proliferative inflammatory processes, among which inflammatory cells such as lymphoids appear. Examination of the media layer of the aortic wall revealed that the smooth muscle cells in it were hypertrophied, the nuclei were hypertrophied and hypeorchromasia, and some were sharply enlarged (Fig. 6). It is determined that connective tissue cells proliferate and inflammatory cells appear along the elastic fibers between the muscle bundles.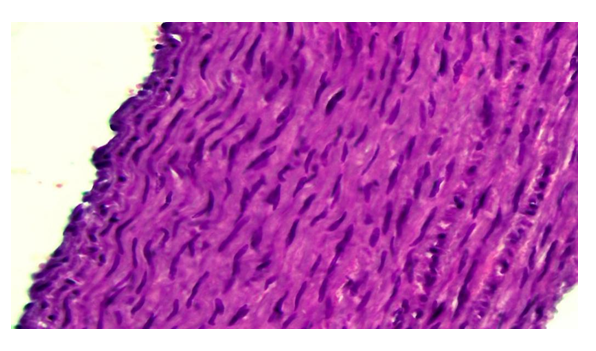 | Figure 6. Corrected group with ASD, 24-month period.Hypertrophy of smooth muscle cells in the aortic wall media. Paint: G-E. X: 10x40 |
The results of morphometric examinations of the animal aorta after irradiation and its correction with an antiseptic stimulant Dorogova (ASD) showed that. The results of morphometric studies showed that in the control group, the intimate thickness of the aorta in 6-month-old animals was 6.87 ± 0.27 μm, but under the influence of radiation it thickened to 11.56 ± 0.35 μm and increased to 8.43 ± 0.24 μm after treatment. mkm was observed. In the untreated group, the reason for the thickening of the intimacy was tissue swelling, dystrophic changes under the influence of radiation, thinning of tissue structures, thickening of the intima. After treatment, these pathological processes stabilized and the tissue became thinner as it returned to its original state. In the later periods of the experiment, i.e., at 12 and 24 months of age, the thickening of the intima continued, with controls being 7.92 ± 0.33 and 8.24 ± 0.36 μm, while after irradiation it was 12.34 ± 0.54 and 10.32 ± Thickening to 0.46 μm, thinning to 10.26 and 9.54 μm after treatment. If the middle aortic wall layer is dynamic, ie at 6 months - 49.57 ± 4.08, at 12 months - 57.2 ± 4.16, and at 24 months - 69.69 ± 2.4 microns, its thickening Less thickened at 6 months (52.86 ± 4.11 μm), relatively more at 12 months (68.16 ± 4.16 μm), and even more thickened at 24 months (81.76 ± 3.4 μm), and after treatment stabilized and came close to the indicators of the control group, ie as follows: 50.65 ± 3.12, 64.28 ± 3.87, 79.26 ± 3.6. It has been confirmed that the thickening of such aortic wall layers under the influence of radiation is certainly due to pathomorphological changes in the tissue. That is, swelling, swelling, cell and fiber dystrophy in all layers of the aortic wall occurred as a result of the addition of inflammatory and calcinous processes in the latter period. After treatment, however, these pathological processes reversed and the thickness of the layers approached the norm. It was observed that the morphometric parameters of the adventitious layer of the aortic wall were almost unchanged under the influence of radiation, and even slightly thinned at 6 and 12 months of age. It was found that after treatment, the adventitious layer of the aorta changed significantly morphometrically, i.e., it thickened (Table 1). 4.45, and at 24 months - 92.28 ± 3.19 μm, an average of 8 μm at 6 months after irradiation, 13.5 μm at 12 months, and 15.5 μm at 24 months, 6 months after treatment - 72.53, 91.12 at 12 months and 107.23 μm at 24 months (Table 1).Table 1. Control of the thickness of the layers of the wall of the Aorta, indications after irradiation and treatment, mkm
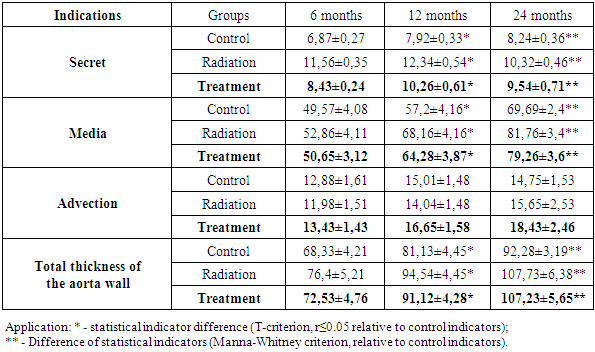 |
| |
|
Subsequent morphometric examinations were devoted to the calculation of the diameter and area of the aortic wall and cavity after irradiation and treatment. In the first period of the experiment, i.e. in 6-month-old animals, the outer diameter of the aorta was enlarged relative to the control group (2.4, ± 0.04 mm) (Table 2) and was 2.8, ± 0.06 mm, and 2.6 ± 0 after treatment. , 07 μm, thickened from 3.3 ± 0.06 mm to 4.2 ± 0.07 mm at 12 months and 4.4 ± 0.05 mm to 5.1 ± 0.07 mm at 24 months after treatment. Decreases were observed to 8 ± 0.08 and 4.8 ± 0.09 μm (Table 2). In contrast, the internal diameter of the aorta was found to decrease in the dynamics of the experiment, the reason being, of course, the thickening of the aortic wall. During the 6-month period of the experiment, it was 1.8 ± 0.03 mm in the control group, 1.6 ± 0.03 mm after irradiation, 1.9 ± 0.05 mm after treatment, and 2.6 ± 0 at 12 months. It was found to decrease from 05 mm to 2.3 ± 0.06 mm, and at 24 months from 3.6 ± 0.07 mm to 3.3 ± 0.08 mm. Calculations of aortic wall and cavity area showed that the aortic wall area in the 6-month period of the experiment was 0.36 mm2 in the control group, expanded to 0.42 mm2 after irradiation, and 1.13 mm2 after treatment, from 0.49 to 0.56 in the 12-month period. to 2.83mm2 after treatment and from 0.64 to 0.72 mm2 at 24 months, and 3.79mm2 after treatment. In the experimental dynamics, it was confirmed that the expansion of the aortic wall area led to a narrowing of the cavity area. During the 6-month period, the aortic cavity area narrowed from 2.54 mm2 to 2.14 mm2 in the control group, from 5.74 mm2 to 4.64 mm2 during the 12-month period, and from 11.33 mm2 to 9.53 mm2 at 24 months. It was found to expand again after treatment: 2.83, 5.31, 10.74 mm2. As a result, it was found that the Wogenworth index was higher in the control group during all periods of the experiment (Table 2).Table 2. Morphometric parameters of the aorta after irradiation and treatment (M ± m), in mm
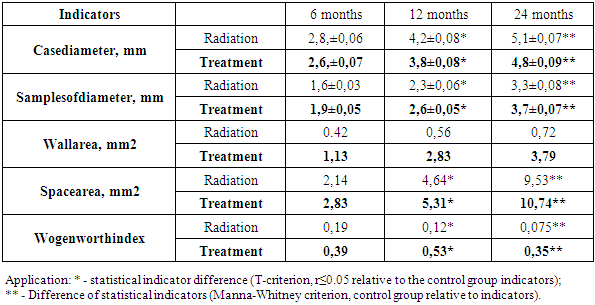 |
| |
|
4. Conclusions
Under the influence of radiation, it was observed that the intimacy of the aortic wall layers thickened from the first period of the experiment, and the thickness of the intima and media layers approached the control group after the treatment procedure.In the dynamics after irradiation, it was observed that the area of the aortic wall thickened and the area of the cavity relative to it decreased, and after treatment, the area of both the wall and the cavity of the aorta expanded sharply.
References
| [1] | Averkin N.S. et al. Morphometric parameters of the aorta in rabbits of different ages and in the conditions of chronic stress // Cardiovascular therapy and prophylaxis. 2012. № 5, p. 132-138. |
| [2] | Averkin N.S., Fedorova M.G., Latynova I.V., Kharitonov E.A., Pivovarov E.V. Mikrometricheskie izmeneniya stenok krupnyx arteriy v usloviyax narushennogo uglevodnogoobmena // Izvestiya vysshix uchebnyxzavedeniy. Povoljskiyregion. Medicinal sciences. 2019. №2. S.144-151. |
| [3] | Drapkina O.M. Sosudistyy vozrast kakfaktorriskaserdechno-sosudistyx. Zabolevaniy // Arterialnaya hypertension. 2014. №20 (4). C.224-231. |
| [4] | Dynamics of indicators of immune status in patients with nonspecific aortic arteritis on the background of combined therapy. Medicinal news. - Minsk, 2012. - №7 (214). - S.78-80. |
| [5] | Kausova G.K., Toleu E.T., Kodasbaev A.T., Nurbakyt A.N. K voprosu profilaktikiserdechno-sosudistyx zabolevaniy // Vestnik KazNMU. 2017. №4. S.40-42. |
| [6] | Strajesko I.D., Akasheva D.U., Dudinskaya E.N., Tkacheva O.N. Starenie sosudov: osnovnye priznaki i mekhanizmy // Kardiovaskulyarnayaterapiya i profilaktika. 2012. №11 (4). S. 93-100. |
| [7] | Carallo C., Tripolino C., De Franceschi M.S., Irace C., Xu X.Y., Gnasso A. Carotidendothelial shear stress reduction with aging is associated with plaque development in twelve years. Atherosclerosis. 2016. vol.251. P.63-69. |
| [8] | Kwak B.R., Back M, Bochaton • Piallat M.L. et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014. vol. 35. P. 13–20. |
| [9] | Pikilidou M., Yavropoulou M., Antoniou M., Yovos J. The contribution of Osteoprogenitor cells to Arterial stiffness and hypertension. J. Vasc. 2015. vol. 52. P. 32-40. |
| [10] | Wu H.H., Wang S. Strain differences in the chronic mild stress animal model of depression. Behav. Brain. Res. 2010. vol. 213. no. 1. P. 94-102. |
| [11] | Abdullaeva Muslima Ahadovna.Pathomorphological Changes that Develop in the Wall of the Aorta Under the Influence of Radiation. CENTRAL ASIAN JOURNAL OF MEDICAL AND NATURAL SCIENCES Volume: 02 Issue: 04 | Jul-Aug 2021 ISSN: 2660-4159. http://cajmns.centralasianstudies.org. |
| [12] | Abdullayeva Muslima Ahadovna. Method for determining morphological and morphometric changes in the aorta after treatment of asd.synergy: journal of ethics and governance. Volume: 01 Issue: 05 | 2021 ISSN: 2181-2616. |
| [13] | Abdullayeva M. A. Sultonova L.J. Cellular factellular factors of endothelial development dysfunctions at NAA. Evropean journal of pharmaceutical and medical research issn 2394-3211 impact factor: 6.222 icv-79.57. |








 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
