-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(2): 179-182
doi:10.5923/j.ajmms.20221202.26
Received: Jan. 9, 2022; Accepted: Feb. 8, 2022; Published: Feb. 24, 2022

Ecdisten and Ecsumid as Effective Means of Preventing Negative Shifts in Certain Metabolic Processes of the Myocardium, Caused by Immobilization Stress
Syrov V. N.
Institute of Plant Chemistry Named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Syrov V. N., Institute of Plant Chemistry Named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The preparation of ecdisten, created based on the phytoecdysteroid 20-hydroxyecdysone, isolated from the rhizomes of Rhaponticum carthamoides and ecsumid (total ecdysteroid-containing drug, including 20-hydroxyecdysone, turkesterone, cyastron, etc.), obtained from the aerial part of Ajuga turkestanica was used in experiment.When it was administered to rats, exposed to prolonged immobilization stress, they effectively maintain the energy balance of the myocardium and prevent negative changes in the antioxidant and NO-ergic systems, observed under these conditions. In terms of the severity of its protective action on metabolic processes in the myocardium or stress, ecsumid surpasses the corresponding action of ecdisten. The study is an experimental animal study. The abilities of ecdisten and ecsumid as agents capable of preventing negative shifts in some metabolic processes of the heart muscle of rats developing under prolonged immobilization stress have been determined.
Keywords: Phytoecdysteroids, Ecdisten, Ecsumid, Immobilization stress, Myocardial metabolism
Cite this paper: Syrov V. N., Ecdisten and Ecsumid as Effective Means of Preventing Negative Shifts in Certain Metabolic Processes of the Myocardium, Caused by Immobilization Stress, American Journal of Medicine and Medical Sciences, Vol. 12 No. 2, 2022, pp. 179-182. doi: 10.5923/j.ajmms.20221202.26.
1. Introduction
- In recent years, it has been established that phytoecdysteroids are widespread in the plant kingdom. They are found both in lower plants: algae, fungi, and in higher ones: ferns, gymnosperms and angiosperms [1,2,3]. Numerous pharmacological studies on various experimental animals have shown that these compounds have the ability to increase the adaptive capacity to stressful environmental factors due to an optimizing effect on metabolic processes in their body in the complete absence of any toxic effects [2,4-9].As a result, on the basis of phytoecdysteroids, drug preparations and biologically active food additives (ecdisten, ecsumid, serlisten, etc.) were created, which have a general strengthening and adaptogenic effect [10,11,12,13,14]. Considering that one of the most vulnerable systems of the body to the negative impact of stress (especially strong and long-term) is the cardiovascular system [15,16]. It seemed extremely important to determine the possibility of using ecdysteroid-containing agents to counteract this process, which is fraught with the subsequent development of diseases such as ischemic heart disease, myocardial infarction and others [15,17,18]. In this regard, this work presents the results of a study of two ecdysteroid-containing drugs - ecdisten and ecsumid.Aim of the research. To determine the ability of ecdisten and ecsumid as a means of preventing negative changes in some metabolic processes of the rat cardiac muscle that develop under prolonged immobilization stress.
2. Materials and Methods
- We used an ecdisten preparation containing purified ecdysterone (20-hydroxyecdysone), isolated from the rhizomes of Rhaponticum carthamoides (Willd.) Iljin and a total ecdysteroid-containing preparation ecsumid (the composition includes 20-hydroxyecdysone, turkesterone, casterone, etc.), obtained from the aerial part of Ajuga turkestanica (Rgl.) Brig [19,20,21]. We have used immobilization of male rats (130–140 g) as a model of severe long-term stress in the supine position for 16 hours [22], obtained from the vivarium at the Department of Pharmacology and Toxicology of the Institute of Chemistry of Plant Substances of the Academy of Sciences of the Republic of Uz. The contents and manipulations carried out with them corresponded to generally accepted international standards for the treatment of laboratory animals (Directive 2010/63/EU of the European Parliament. September 22, 2010). Ecdisten and Ecsumid were administered per os in the form of an aqueous emulsion with gummi arabicus (due to the poor solubility of the preparations) at a dose of 5.0 mg / kg (in preliminary experiments it was established as the most optimal), immediately before fixation of the animals. Control animals received an adequate amount of an aqueous emulsion of gummi arabicus. The animals were slaughtered by instant decapitation. A number of indicators assessed post-stress changes in myocardial metabolism. The content of glycogen was determined according to [23], adenine nucleotides according to [24], creatine phosphate according to [25] data. The energy charge was calculated using the formula: (adenosine triphosphate + 0.5 adenosine diphosphate) / sum of adenine nucleotides [26]. The content of malondialdehyde was determined according to [27], the activity of catalase according to [28] and superoxide dismutase according to [29] data. The activity of endothelial NO-synthase [30] and the main metabolites of nitric oxide [31] was also determined. The obtained data were subjected to statistical processing using the Student’s t-test.
3. The Results of Research
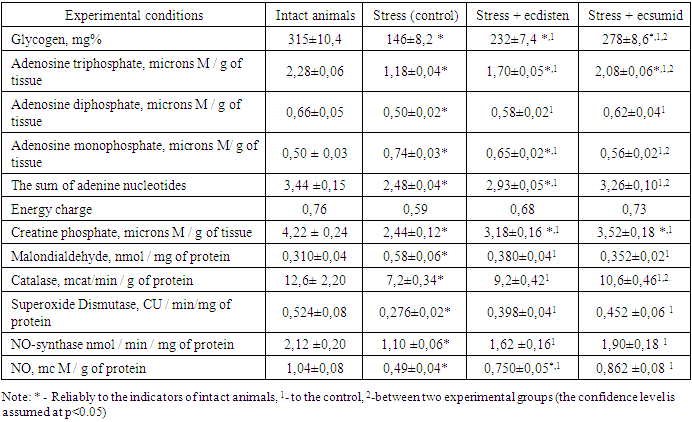
- As can be seen from the presented table, prolonged immobilization stress was accompanied by pronounced disorders of metabolic processes in the myocardium. The negative change in this case of energy metabolism was evidenced by the fact that stressful rats showed a lower preservation of the glycogen fund in the heart muscle, combined with a lower preservation of the adenosine triphosphate and creatine phosphate fund, as well as a significantly lower energy charge. The content of glycogen, ATP and creatine phosphate in control animals was lower than in intact animals by 53.7; 48.3 and 42.2%, respectively. The calculated value of the energy charge was 22.4% lower than the corresponding indicator in intact animals. The revealed changes seem to be very negative manifestations of the stress impact on the animal organism and the myocardium, in particular, since it is the decrease in the energy potential of cardiomyocytes that largely impairs their functional activity under these conditions [15]. In addition, free radical reactions increased in the heart, which play a significant role in myocardial damage during stress [13,15]. The products of these reactions, attacking unsaturated fatty acid residues of membrane phospholipids, change their polarity. This leads to disruption of the activity of enzymes, receptors, channel-forming proteins located on or inside membranes. As a result, the phospholipid layer becomes permeable to most cations (Ca2+, Na+), the accumulation of which in cardiomyocytes has a damaging effect [16].Thus, under conditions of reproducible stress, an increase in the level of malondialdehyde, one of the products of lipid peroxidation, was revealed by 87.1%, which, apparently, could be due to a depression of the body’s antioxidant defense by 47.3. In addition, catalase decreased the activity of superoxide dismutase by 42.9% (table 1).
|
4. Conclusions
- 1. Ecdisten and ecsumid in experiments on rats exposed to prolonged immobilization stress prevent negative changes in the metabolic processes of the myocardium.2. By its ability to maintain homeostasis of energy production under stress, to optimize the state of the antioxidant and NO-ergic system of the heart muscle, ecdisten is inferior to the total ecdysteroid-containing drug ecsumid.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML