-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(2): 78-83
doi:10.5923/j.ajmms.20221202.02
Received: Dec. 31, 2021; Accepted: Jan. 24, 2022; Published: Feb. 15, 2022

Structure of Chronic Myeloleukemia Patients in Uzbekistan: Analysis and Perspectives
Sayyorakhon Saidusmonovna Saydamanova1, Abdurakhmon Abdumavlyanovich Kayumov2
1Clinical Department, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz
2Administration, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz
Correspondence to: Sayyorakhon Saidusmonovna Saydamanova, Clinical Department, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to systematize, structure and present information on the age and sex structure of chronic myeloleukemia (CML) patients and the long-term results of therapy with tyrosine kinase inhibitors (TKIs). The study enrolled 1033 patients diagnosed with CML who were treated with tyrosine kinase inhibitors of first generation (Glivec (imatinib) and 42 patients who were treated with the second generation (Tasigna (nilotinib)) from different regions of Uzbekistan. The carried out work will allow to replenish the register with the obtained data, and the analysis of patients' structure. Also this study to form and develop the register of patients with CML and oncohematological patients in general.
Keywords: Chronic myeloleukemia (CML), Tyrosine kinase inhibitors (TKIs), Glivec, Tasigna, Sex and age, Regional, Registry
Cite this paper: Sayyorakhon Saidusmonovna Saydamanova, Abdurakhmon Abdumavlyanovich Kayumov, Structure of Chronic Myeloleukemia Patients in Uzbekistan: Analysis and Perspectives, American Journal of Medicine and Medical Sciences, Vol. 12 No. 2, 2022, pp. 78-83. doi: 10.5923/j.ajmms.20221202.02.
Article Outline
1. Introduction
- The use of tyrosine kinase inhibitors (TKIs) in the treatment of chronic myeloleukemia (CML) patients has increased the survival rate [1-5]. Sufficiently long history of TKIs use in Uzbekistan allows us to make certain observations and conclusions about the effectiveness and long-term effects of the therapy, which raises the question of the need to streamline the data accumulated over a long period of time. The creation of a registry of patients with CML who have been treated with TKIS will allow further improvement of treatment protocols and their effectiveness [8-13].
2. Main Body
2.1. The Purpose of Our Research
- To systematize, structure and present information on the sex and age structure of patients with CML and the long-term results of therapy with tyrosine kinase inhibitors (TKIs).
2.2. Material and Methods of Study
- The study included 1033 patients with established diagnosis of CML who received first generation (Glivec (imatinib)) tyrosine kinase inhibitors and 42 patients who received second generation (Tasigna (nilotinib)) drugs from different regions of the Republic of Uzbekistan. Patients with the presence of Philadelphia chromosome (Ph+) and/or the presence of BCR-ABL transcript confirmed by cytogenetic method were selected for the study [7].We studied the structure of patients, their distribution by sex and age. A subgroup of patients with CML treated with Glivec included 991 patients, of whom 520 were men and 471 women. Another part of patients with CML received the second-generation TKIs - Tasigna. The subgroup of patients with CML treated with "Tasigna" included 42 patients, including 24 men and 18 women. Retrospective studies of treatment efficacy were performed. Statistical processing was performed using an online calculator [14].
2.3. Results of the Study
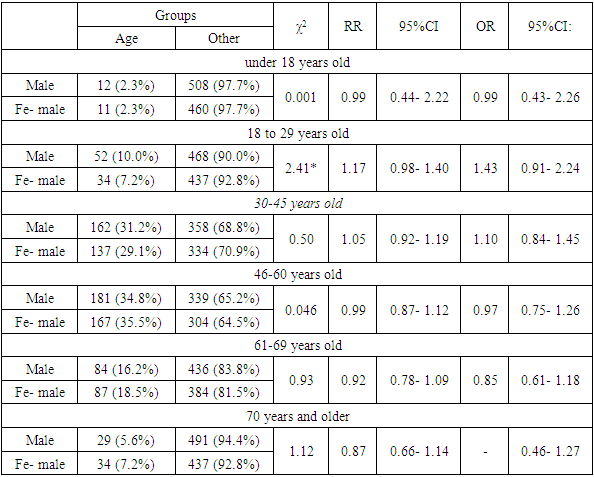
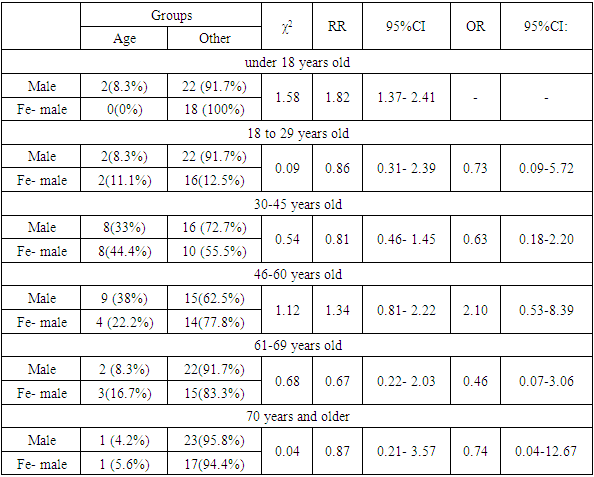
- The sex and age structure of the groups of patients treated with the first (Glivec) and second (Tasigna) generations of TKIs drugs was studied (Fig. 1-2; Table 1-2).As we can see from Figure 1, patients of the middle age category prevailed among the patients. The largest proportion were patients from 30 to 45 and 46 to 60 years old. The data presented in the table indicate the absence of statistically significant differences between male and female patients in all age categories studied among the patients who took Glivec.
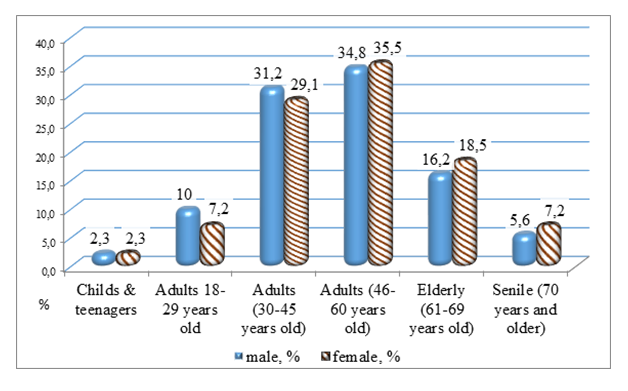 | Figure 1. Gender and age structure among patients treated with “Glivec” |
|
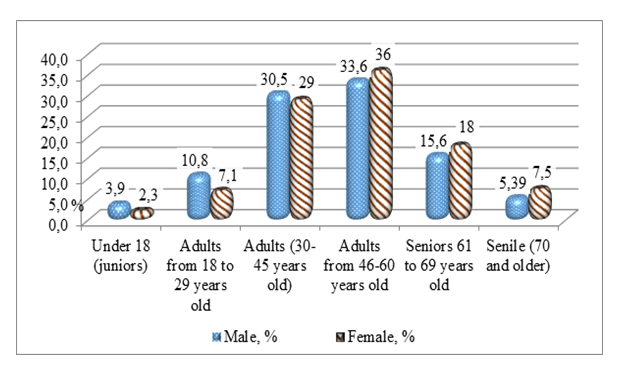 | Figure 2. Age and sex structure among patients who received "Tasigna" |
|
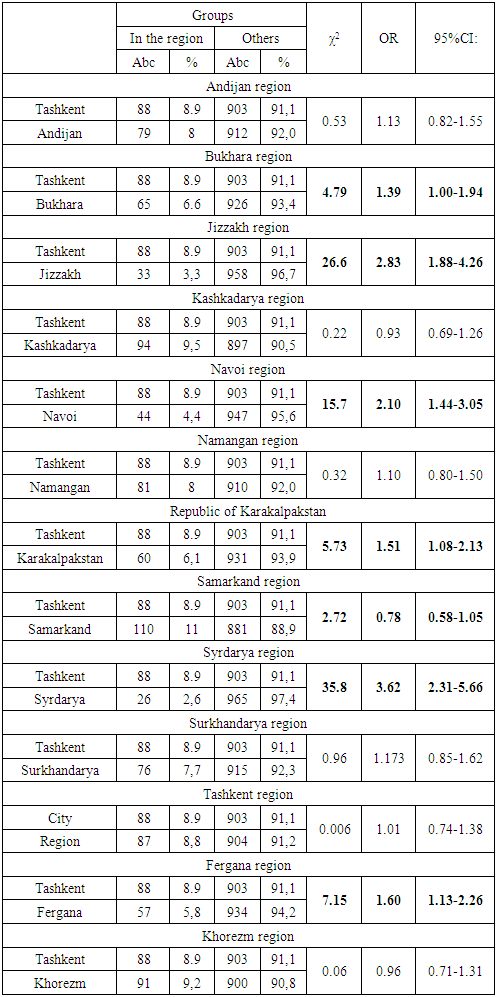
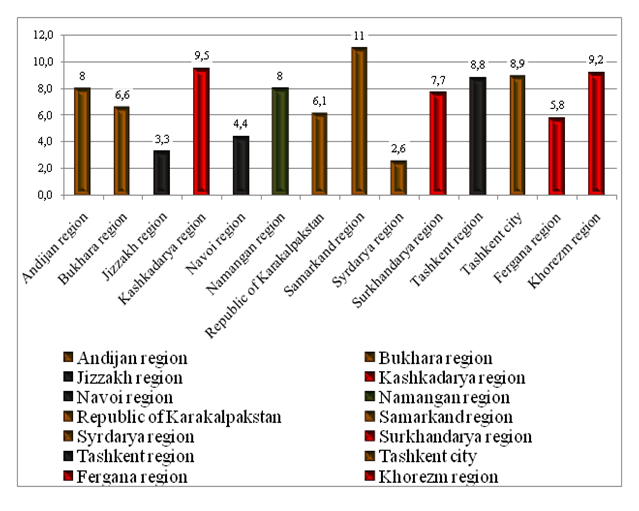 | Figure 3. Structure by regions (regions) of patients who received Glivec |
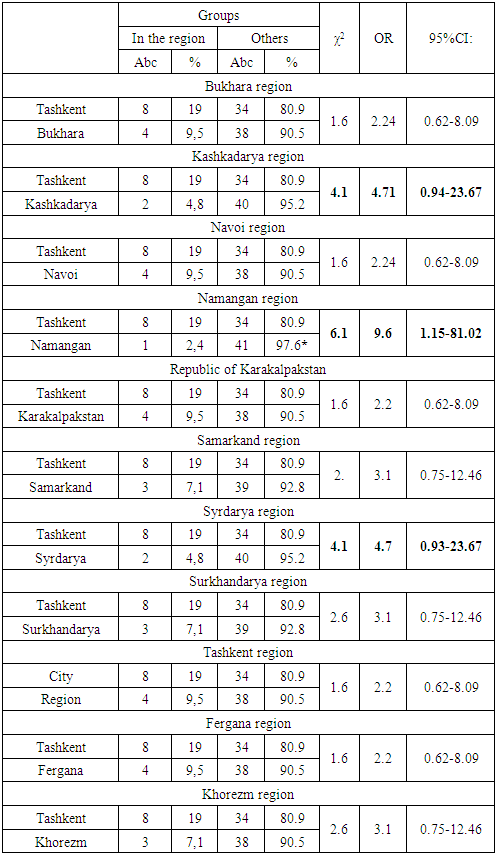
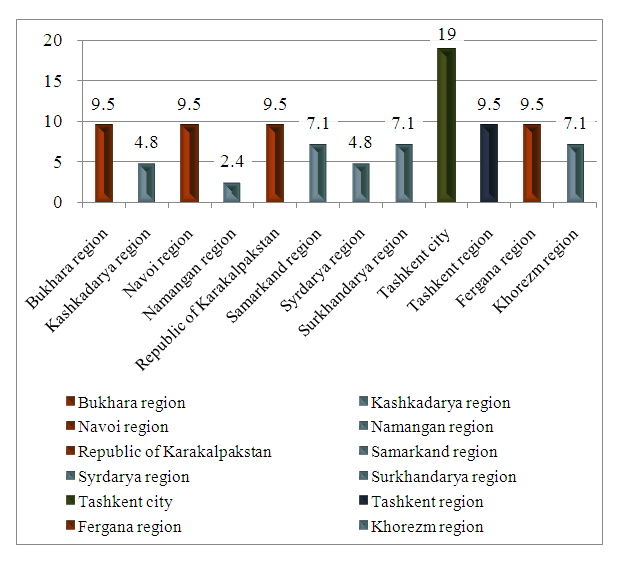 | Figure 4. Structure by region among patients with CML who received Tasigna |
|
|
3. Conclusions
- The conducted work will allow to replenish the register with the data obtained, and the analysis of the structure of patients will allow to expand the understanding of the structure of patients, to give a full assessment of the long-term results of treatment of this category, to form and develop a register of patients with CML and oncohematological patients in general. The formation of such register and its support at the state level will allow to visualize the structure of the study group, monitor the effectiveness of CML therapy and improve its efficiency. This is especially relevant, in order to improve the quality and longevity of CML patients, in particular the long-term survival rate [7-9].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML