-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(1): 66-70
doi:10.5923/j.ajmms.20221201.13
Received: Jan. 11, 2022; Accepted: Jan. 21, 2022; Published: Jan. 27, 2022

Preinfarction Angina Pectoris as Protection Against Reperfusion Injury of the Myocardium in Patients with Acute Coronary Syndrome with ST-Elevation
A. L. Alyavi, A. I. Aminov, A. K. Koirov
Republican Research Centre of Emergency Medicine, Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The undoubted benefit of coronary blood flowearly restoration in acute myocardial infarction can bring additional reperfusion injury of cardiomyocytes. In this regard, it is urgent to search for ways to protect the myocardium in conditions of ischemia and early reperfusion in order to prevent additional myocardial injury. Currently, there are numerous literature data confirming that preinfarction angina can manifest a cardioprotective effect during reperfusion therapy. Our study showed that in patients with preinfarction angina pectoris who underwent early myocardial revascularization, reperfusion arrhythmia, repeated ischemic events, acute left ventricular failure occurred much less common. Also, due to its protective properties, preinfarction angina contributed to the reduction of ischemic and reperfusion damage to the myocardium, limiting the development of myocardial necrosis.
Keywords: Acute coronary syndrome with ST-elevation, Reperfusion myocardial injury, Preinfarction angina, Ischemic preconditioning
Cite this paper: A. L. Alyavi, A. I. Aminov, A. K. Koirov, Preinfarction Angina Pectoris as Protection Against Reperfusion Injury of the Myocardium in Patients with Acute Coronary Syndrome with ST-Elevation, American Journal of Medicine and Medical Sciences, Vol. 12 No. 1, 2022, pp. 66-70. doi: 10.5923/j.ajmms.20221201.13.
1. Introduction
- According to experts of the World Health Organization (WHO), an acute ST-elevation myocardial infarction (STEMI) is currently the leading cause of death and disability worldwide. Hospital mortality from STEMI makes up 8-12% [1-3]. The introduction into clinical practice of modern methods of treatment such as systemic and selective intracoronary thrombolysis, percutaneous transluminal coronary angioplasty (PTCA), stenting of the infarct related artery (IRA), coronary artery bypass grafting has led to an improvement in the survival of STEMI patients by limiting the necrosis zone [1,4,3,5-7]. However, performing revascularization, the phenomenon of reperfusion may be accompanied by an increase in myocardial injury, the so-called reperfusion injury (RI) of the myocardium [2,6,8]. The main pathogenesis of myocardial RI is excessive formation of free oxygen radicals (after reoxygenation) with activation of lipid peroxidation (LP) of membranes, uncontrolled entry of calcium ions and their excessive accumulation in cardiomyocytes, leading to disruption of the energy supply of the cell due to inhibition of ATP production [9-12]. Therefore, in clinical practice more and more attention has been paid to the metabolic protection of the myocardium from ischemic reperfusion injury, especially in patients with STEMIfor the last decade. One of such directions is preventive cardioprotection (different variants of myocardial preconditioning) and therapeutic approaches (metabolic therapy carried out after ischemia and early reperfusion period - myocardial postconditioning) [13,4,3,12,14]. This trend began to develop actively after the publication in 1986 of the studies of R Murry CE, et al., which in animal experiments showed that inducing episodes of short ischemia and myocardial reperfusion before or after a period of prolonged myocardial ischemia could significantly reduce myocardial injury. This phenomenon is called ischemic preconditioning (IP) and postconditioning (adaptive preparation of the heart to counteract damaging effects) [12]. Later, a number of authors demonstrated the cardioprotective effect of IP in an experimental study on the myocardium of dogs. The induction of short-term (5 min) episodes of myocardial ischemia, before prolonged (more than 40 min) occlusion of the coronary artery with subsequent reperfusion led to a limitation of the necrosis size.According to the literature data, basically the mechanism of the IP phenomenon is three consecutive processes: to the response of the initial ischemic stimulus, activation of IP triggers (adenosine, bradykinin, nitric oxide, etc.), which interact with the receptors of cardiomyocytes and vascular endothelium, which leads to the launch of an intracellular cascade of signal transduction which offers protective effects. The final effect of the cascade is the opening of ATP-dependent potassium channels and the closure of Ca2+-dependent mitochondrial pore (pore that changes the permeability of the mitochondrial membrane (mPTP)). As a result, with subsequent more pronounced ischemia, the metabolic activity of the myocardium decreases, the rate of ATP breakdown decreases and glycogenolysis slows down, which lead to an increase in myocardial resistance to ischemia [15-16,12,8]. However, this hypothesis (cardioprotection technique) has been confirmed only in laboratory conditions, attempts to transfer laboratory experience into clinical practice still remain unsuccessful. In clinical conditions, the existing preinfarction angina can act as a phenomenon of ischemic preconditioning. As many authors suggest, the existing preinfarction angina can protect the myocardium from necrosis during reperfusion therapy in patients with STEMI.Aim of the study was to evaluate the effect of preinfarction angina on reperfusion injury of the myocardium and on the clinical course of an acute ST-elevation myocardial infarction (STEMI) in patients undergoing thrombolytic therapy.
2. Material and Methods
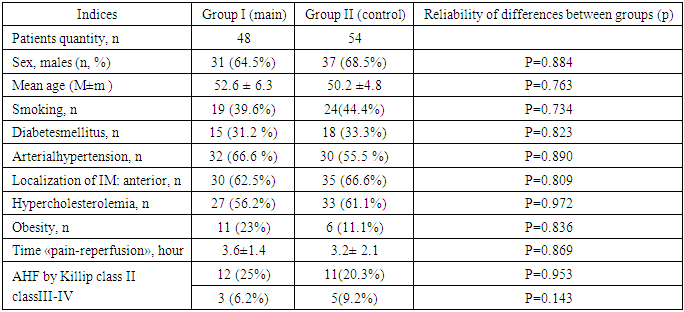
- 102 patients with acute coronary syndrome with STEMI at the age from 25 to 70 years (mean age 53.4 ± 6.3 years) were examined and hospitalized in the first 6 hours after the onset of the disease symptoms. Inclusion criteria in the study: typical clinical picture of ACS, presence of ST-elevation of more than 2 mm in two adjacent thoracic leads or ST-elevation of more than 1 mm in standard leads; acute blockade of the left leg Gis bundle. Exclusion criteria: age over 70 years; pain syndrome more than 6 hours; history of myocardial infarction (MI); patients with severe concomitant somatic diseases (oncological, mental, surgical, acute cerebral circulation disorders, diabetes mellitus in the decompensation stage, chronic obstructive bronchitis in the exacerbation stage). All patients underwent basic treatment: antiplatelet agents, anticoagulants, beta-blockers, ACE inhibitors, nitrates and statins. In the presence of indications, diuretics, narcotic analgesics, antiarrhythmic agents were used. Thrombolytic therapy was performed using streptokinase 1.5 million units intravenous drip for 40 minutes. Life-saving percutaneous transluminal coronary angioplasty (PTCA) after unsuccessful thrombolysis for 12 hours was performed in 3 (6.2%) patients in the main group and in 7 (13%) patients in the control group. Two groups of patients were formed by random sampling, who had no statistically significant differences in the initial clinical and anamnestic data and the basic therapy used: Group I (main) – 48 patients who had angina attacks (preinfarction angina) during the last 24-48 hours before the development of MI; Group II (control) consisted of 54 patients in whom MI developed suddenly without any preinfarction angina. Table 1 shows the main clinical and anamnestic data of patients.
|
3. Results
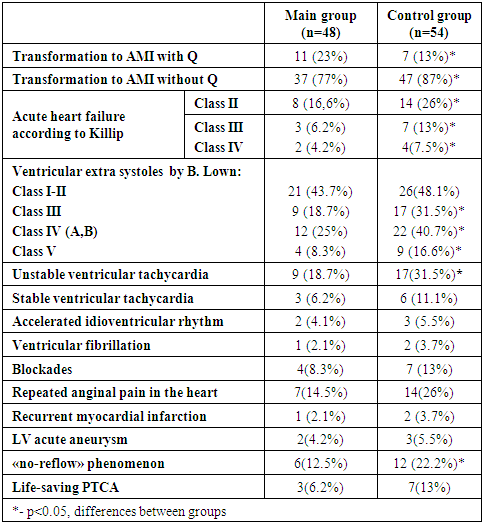
- The initial characteristics of the patients in the treatment groups were comparable in all clinical, anamnestic, demographic indicators and in the time of "pain-reperfusion". The analysis of tissue reperfusion showed a faster decrease in the ST segment (by 50/70% of the initial one 180 minutes after thrombolysis) in patients with preinfarction angina (main group) in compare with the control one (29(60.4%) versus 28(51.8%), respectively, (р <0.05)). Ineffective tissue reperfusion "no-reflow" was observed in 6 (12.5%) patients of the main group and in 12 (22.2%) patients of the control group (p <0.05). Taking into account the persistent anginal pain in the heart for 12 hours after reperfusion and the absence of signs of tissue reperfusion on the ECG, life-saving PTCA was performed in 3 (6.2%) patients in the main group and in 7 (13%) patients in the control group (p >0.05).Reperfusion ventricular arrhythmias (the first day of reperfusion) were significantly less common in patients with preinfarction angina (main group). In patients of the main group, single ventricular extra systoles (VES I-II class according to V. Lown) were found slightly less (43.7% versus 48.1%, p >0.05). Life-threatening ventricular arrhythmias, VES of high grades (III-V class according to V. Lown) were statistically significantly less frequently recorded in patients of the main group, compared with the control group (p <0.05). There were also differences in the occurrence of the number of episodes of unstable and stable ventricular tachycardia (VT). After reperfusion on the first day, unstable VT was recorded in 9 (18.7%) patients in the main group, and in 17 (31.5%) patients in the control group (p <0.05). There were also slightly fewer episodes of sustained VT in the main group compared to the control group (in 6.2% of patients versus 11.1%, p >0.05). In one case of the main group and in 2 cases of the control group VT transformed into ventricular fibrillation (VF) which in all cases were successfully stopped by defibrillation. Accelerated idioventricular rhythm (AIVR) in both groups occurred mainly in the early reperfusion period. There were no significant differences in the incidence of AIVR in the comparison groups (Table 2). Also, transient cardiac conduction disturbances (AV-blockade, intraventricular blockade) in the study group were slightly less than in the control group (in 8.3% of patients versus 12.9%, p >0.05).
|
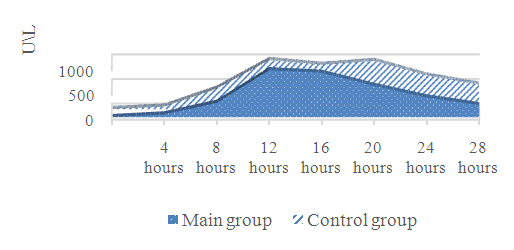 | Figure 1. Dynamics of CPK-MB, U/L |
4. Discussion
- It is known that recanalization of an infarct-related coronary artery (IRCA) in STEMI patients also contributes to myocardial reperfusion injury [1,10,3]. It led to the development of new views in the search for new methods of prevention and protection of the myocardium during reperfusion therapy in patients with STEMI. One of these methods was proposed by R. Lange et al. (1984), who, as a result of their experimental studies, proved that by depleting ATP after repeated short episodes of ischemia, it is possible to adapt the myocardium and make it more resistant to prolonged ischemia, thereby it is possible to reduce the zones of myocardial necrosis - ischemic pre-conditioning (IPC) [2-3,11,15]. In clinical practice preinfarction angina is the equivalent of IPC [1,12]. There are a number of clinical studies which show that previous attacks of angina pectoris during the development of AMI can have a cardioprotective effect on the myocardium if they occur within 24-48 hours before AMI [1,4,10,12]. One of the first such studies is the TIMI-4 (Trombolysis in Myocardial Infarction -Phase 4) and GUSTO-I (Global Utilization of Streptokinase and t-PA for Occludedcoronaryarteries-I). According to these studies, patients with a history of exertional angina pectoris may be better able to tolerate myocardial infarction than patients who have not had previous angina attacks: in patients with preinfarction angina (PIA), the size of the MI focus is smaller, the increase in the activity of marker enzymes (CPK-MB) is less pronounced, the contractile function of the myocardium is better preserved, severe heart failure and reperfusion life-threatening arrhythmias are developed less often due to myocardial reperfusion during the hospital period of treatment. The results also showed that patients with a previous attack of exertional angina compared with patients without prior PIA had a rapid onset of reperfusion in the infarct-associated coronary artery. This had a positive effect on the clinical course of myocardial infarction: reperfusion life-threatening arrhythmias were observed much less frequently, attacks of acute heart failure and repeated anginal pain in the heart were much less common, a smaller size of the necrosis focus, assessed by the activity of the MB-fractions of creatine phosphokinase and graphically the area under the curve.Thus, our results are consistent with numerous literature data and indicate the cardioprotective effect of short-term episodes of preinfarction angina in STEMI patients undergoing reperfusion therapy (TLT). Short repeated episodes of ischemia / reperfusion appear to be protective, adapting the myocardium to subsequent severe, prolonged ischemia / reperfusion, and is a spontaneous factor in the protection of the myocardium from ischemic-reperfusion injury, has a positive effect on the size of myocardial infarction, restoration of LV contractile function, the effectiveness of TLT, and the development of reperfusion life-threatening arrhythmias.
5. Conclusions
- The presence of preinfarction angina in patients with STEMI has a positive effect on the effectiveness of reperfusion therapy, accelerating the "symptom-reperfusion" time. Preinfarction angina helped to reduce the focus of ischemic-reperfusion injury of the myocardium due to its protective properties, limiting the development of myocardial necrosis, and also reduced the development of life-threatening reperfusion arrhythmias. In patients with PIA, the clinical course of myocardial infarction is smoother; attacks of acute heart failure and repeated anginal pain in the heart during the hospital period of treatment are much less common.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML