-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(1): 40-45
doi:10.5923/j.ajmms.20221201.08
Received: Jan. 2, 2022; Accepted: Jan. 22, 2022; Published: Jan. 24, 2022

May-Thurner Syndrome Complicated by Deep Vein Thrombosis of Left Lower Extremity: Clinical Study on the Optimal Timing to Perform Stenting
Sardor Kuchkorov1, Tang Li Ming2, Liu Chun Jiang3, Nasritdinov Umid4
1Student of Master's Degree, Shaoxing University, Zhejiang Province, China
2Head of the Vascular and Hernia Department, Shaoxing People’s Hospital, Zhejiang Province, China
3Secretary of the Vascular and Hernia Department, Shaoxing People’s Hospital, Zhejiang Province, China
4Head of the Emergency Surgical Department, 2nd Clinic of the Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Tang Li Ming, Head of the Vascular and Hernia Department, Shaoxing People’s Hospital, Zhejiang Province, China.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

May–Thurner syndrome (MTS) is a rare clinical entity featuring venous obstruction of the left lower extremity. The aim of the present study was to report our experience with MTS complicated by deep vein thrombosis (DVT) and to evaluate the utility of tactically new approach, in which balloon angioplasty and stenting precede catheter- directed thrombolysis (CDT) therapy.
Keywords: May–Thurner syndrome, Deep vein thrombosis, Iliac vein stenting, Catheter directed thrombolysis
Cite this paper: Sardor Kuchkorov, Tang Li Ming, Liu Chun Jiang, Nasritdinov Umid, May-Thurner Syndrome Complicated by Deep Vein Thrombosis of Left Lower Extremity: Clinical Study on the Optimal Timing to Perform Stenting, American Journal of Medicine and Medical Sciences, Vol. 12 No. 1, 2022, pp. 40-45. doi: 10.5923/j.ajmms.20221201.08.
1. Introduction
- May-Thurner syndrome (MTS) or iliac vein compression syndrome is associated with deep vein thrombosis (DVT) resulting from chronic compression of the left iliac vein against lumbar vertebrae by the overlying right common iliac artery. MTS refers to chronic compression of the left iliac vein against the lumbar spine by the overlying right common iliac artery. The compression may be asymptomatic. The syndrome is a clinical spectrum of physical findings and history plus the lesion. It is characterized by the varying degrees of venous hypertension. This can be non-thrombotic, combined with acute DVT or post-thrombotic. Traditionally, acute DVT was treated with standard anticoagulation and sometimes, thrombectomy. However, these measures do not address the underlying culprit lesion of mechanical compression. Furthermore, if managed only with anticoagulation, patients with residual thrombus are at risk for developing recurrent DVT or post-thrombotic syndrome (PTS). Both retrospective and prospective studies have shown that endovascular management should be the preferred approach to dissolve proximal thrombus and also to treat the underlying compression with endovascular stent placement. We propose a tactically new approach, in which balloon angioplasty and stenting precede catheter-directed thrombolysis (CDT) therapy. The results of this approach are studied and presented in this article.
2. The Main Results and Findings
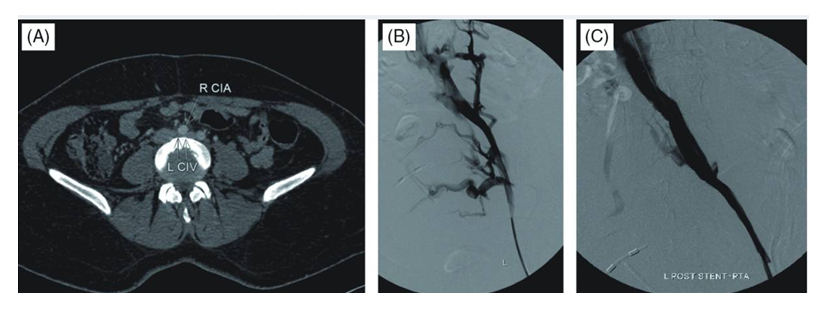
- To investigate the effect of the timing of stent implantation on the curative effect of thrombolytic therapy for acute deep venous thrombosis (DVT) of left lower extremity secondary to May-Thurner syndrome.We retrospectively analyzed data collected between January 2017 and January 2019 on 26 MTS complicated by DVT patients treated using endovascular techniques. We reviewed patient demographics, symptoms, procedural features, radiological and clinical outcomes. Written informed consent was obtained from each patient after the purpose and the risks of treatment were fully explained.All patients exhibited radiographic evidence of iliac vein stenosis caused by MTS upon computed tomography (CT) venography or a combination of duplex ultrasound and venography.Patient selection criteria:- Ultrasound or angiography-confirmed DVT of the left lower extremity; - Onset time ≤14 d; - Luminal stenosis of the left iliac vein >70%;- No history of cerebral haemorrhage, no history of gastrointestinal and other internal organ bleeding within 1 month.Technical success was defined as successful restoration of antegrade flow, with <30% residual stenosis or thrombus. Iliac vein patency was defined as uninterrupted flow apparent on Color Doppler Ultrasound (CDU) or venography. Clinical success was defined as improvement in and resolution of symptoms on follow-up visits.Of our patients, 18 (69.2%) were female and 8 male. Mean patient age was 67.23 ± 7.37 years (range, 18–73 years). All patients were symptomatic. 24 patients presented with acute DVT (16 iliofemoral; eightiliofemoropopliteal); two with subacute DVT (one iliofemoral, one iliofemoropopliteal) chracterized by swelling of and pain in the left lower extremity. Six patients exhibited risk factors for DVT development. These were a previous history of DVT (n = 1); postoperative status (n = 3); postpartum status (n = 1); oral contraceptive (n = 1) and the Factor V Leiden mutation (n = 1). No patient had a symptomatically indicated pulmonary embolism. Onset time was 4 hours~10 days, average 3.65±2.71 days.An antegrade venography of the lower limbs was performed through the left (affected) dorsal vein of the lower extremity to determine the location, extent, degree of obstruction, and collateral circulation of the thrombus. The right (healthy) femoral vein approach was taken 0.5 to 1.0 cm below the renal vein to place the Aegisy disposable filter (Lifetech Shenzhen Technology Co., Ltd.); the patient was in the prone position, and vascular access was established via ultrasonography-guided puncture. After establishment of deep venous guidewire access and introduction of a balloon catheter (diameter 8-10 mm) to expand the deep vein thrombosis in sequence andto crush the thrombus. Subsequently, the left iliac vein was found to be severely narrowed after balloon dilation, and the Wallstent (Boston Technology Company, USA) /SMART (Cordis, US) stent with a diameter of 12 to 14 mm and a length of 80 to 100 mm was implanted into iliac vein, expanded after balloon dilatation. After that we inserted thrombolysis catheter (effective perfusion length 20-50 cm, subject to complete coverage of thrombus), connected to a micro pump, and performed continuous infusion of urokinase (dissolved in 100 mL of 0.9% sodium chloride solution) at 60×104 U/24 h, supplemented with low molecular weight heparin sodium (5 000 U/12 h). After the venous lumen is unobstructed and the thrombolysis was stopped, cava filter was removed through the right femoral vein. Subsequent 24-hour angiography was done to review thrombus clearance: no residual filling defects in the popliteal, femoral, iliac, and inferior vena cava, and stop thrombolysis after the lumen is unobstructed. All patients received 3000 units of heparin at the start of each procedure. Heparin was stopped when the international normalized ratio (INR) reached 2. We maintained the activated partial thrombin time (APTT) at a normal value 2~2.5 times, while adjusting the warfarin medication. Regular warfarin anticoagulant therapy was prescribed for at least 6 months. All patients were instructed to wear graduated compression stockings. The circumference difference (the circumference of crus 10 cm below the knee and the circumference of thigh 15 cm above the knee) was calculated to determine the detumescence rate of affected limb. The detumescence rate of the affected limb was calculated according to the circumference difference of the crus of the affected side and the healthy side and the circumference difference of the thigh of the affected side and the healthy side respectively. The detumescence rate of affected limb = (the circumference difference before treatment-the circumference difference after treatment) / the circumference difference before treatment × 100%. Thrombus clearance rating: Thrombus clearance rate >90% is grade III, 50%~90% is grade II, <50% is grade I, thrombus clearance rate = (pre-treatment score-post-treatment score)/pre-treatment score×100%. We evaluated the veins of the lower extremities (popliteal vein, lower superficial femoral vein, upper superficial femoral vein, common femoral vein, external iliac vein, common iliac vein, inferior vena cava, etc.) according to angiographic images. Complete obstruction is 3 points, subtotal obstruction (50%~99%) is 2 points, partial obstruction (<50%) is 1 point, 0 points for complete patency.During treatment we focused on observation of symptomatic pulmonary embolism and bleeding complications (exudation, congestion, hematoma at the puncture point, bleeding from the gums or bleeding from other organs). Follow-up clinical and imaging evaluations were initially performed at 1, 3, 6 and 12 months, and yearly thereafter. All patients had greater than two-year follow-up. Follow-up imaging featured primarily CDU. A few patients were re-imaged using CT or repeat venography because it was difficult to obtain adequate images of the stents using CDU alone.SPSS 22.0 software was used for data analysis. The counting data were expressed as percentage and compared by chi square test. The measurement data were expressed as mean ± standard deviation (x ± s) and compared by t test. Rank sum test was used for rank data. A P value < 0.05 indicated a significant difference.Venography performed during endovascular treatment of MTS revealed significant stenosis of the left common iliac vein in 18 of 26 (69.2%) patients. In 8 of the 26 (30.7%) patients, total occlusion of the left common iliac vein (LCIV) was evident. All patients presenting with venous varices exhibited common iliac vein stenosis.26 cases were implanted with 26 left iliac vein stents (14 mm×100 mm – 7 cases, 14 mm×80 mm - 13 pieces, 14 mm×60 mm - 1 piece, 12 mm×80 mm - 4 pieces, 12 mm×60 mm - 1 piece), the patency rate of the deep vein and iliac vein stent was 100%. No symptomatic pulmonary embolism occurred. The average dosage of thrombolytic drugs (urokinase) was 3.27±1.41million U. Thrombolysis was performed for 43-169 h (mean 78.69 ± 34.33 h) in 26 patients with acute and subacute DVT. After the treatment, the temporary filters of the inferior vena cava were taken out.
3. Conclusions
- In this study, patients with severe iliac vein stenosis (>70%) were treated according to new tactic: iliac vein stent implantation followed by CDT. Compared with the traditional stent implantation model after CDT, the dosage of urokinasewas significantly reduced, and the indwelling time of thrombolytic catheter was significantly shortened. And there are possible reasons: 1. Implanting a stent to open the iliac vein will help reduce the venous pressure of the lower extremities, which strengthen the diffusion of urokinase from the distal end to the proximal end, and reduce transcollateral reflux;2. Increase blood flow to make more plasminogen, activated by urokinase, thereby enhancing the thrombolytic effect;3. Balloon expansion before stent implantation has an auxiliary effect on thrombolysis.The scientific significance shows that the new model improves the efficiency of thrombolysis while satisfying the curative effect. In addition, the use of the new model has certain advantages: - The iliac vein stent implantation and CDT can be completed in one operation at the same time, which can reduce the number of consumables such as guide wires, catheters, and balloons; - The efficiency of thrombolysis is improved, the number of re-examinations and the dosage of thrombolytic drugs are reduced, and the course of the disease is shortened.The limitations of the present study were the retrospective nature of the work and the limited number of MTS patients evaluated. Follow-up periods and control numbers were not standardized, and follow-up periods were relatively short. Another potential limitation is the variability in stent type and size. No clinical scale was used for symptomatic evaluation; this was a significant limitation.As a result, recent reports have shown that endovascular treatment of symptomatic MTS patients with thrombotic or nonthrombotic presentations is both feasible and safe, and improves symptoms. On long-term follow-up, stent patency does not seem to be affected by ongoing extrinsic compression of a vein by the adjacent iliac artery. We obtained excellent immediate results and early patency, with minimal complications, using localized CDT, angioplasty, and stent implantation.The authors declare that they have no conflicts of interest.MTS – May-Thurner syndromeDVT – Deep vein thrombosisLCIV – Left common iliac veinRCIV – Right common iliac veinRCIA – Right common iliac arteryIVC – Inferior vena cavaCVI – Chronic venous insufficiencyIVCS – Iliac vein compression syndrome CDT – Catheter directed thrombolysisPTS – Post-thrombotic syndromeCT – Computed tomographyCDU –Color Doppler Ultrasound
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML