-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2021; 11(12): 921-925
doi:10.5923/j.ajmms.20211112.16
Received: Nov. 30, 2021; Accepted: Dec. 16, 2021; Published: Dec. 29, 2021

Development Factors of Recurrent Respiratory Papillomatosis
Nadjimutdinova N. Sh., Inoyatova F. I., Abdukayumov A. A., Khegay T. R.
Republican Specialized Scientific Practical Medical Center of Pediatrics, Tashkent, Uzbekistan
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim of the study was to determine the possible relationship between the characteristics of the clinics and the definition of the human papillomavirus with its viral burden. Material and methods. A study of 92 patients with respiratory recurrent papillomatosis was conducted. There were 71 children and 21 adults. The mean age of children was 4.23±2.1, adults – 34.2 years. The patients were performed an endoscopic examination with an assessment of the lesion according to the Derkay scale (2010). The patients also underwent a determination of the human papillomavirus by means of the Real-time polymerase chain reaction and a quantitative study to monitor the human papillomavirus for identifying a possible relationship between viral burden and clinical course (prevalence, recurrence rate). Results. A study of 92 patients was carried out: typifying revealed only HPV types 6 and 11, other genotypes were not detected. Quantitative analysis revealed the presence of human papillomavirus 6, 11 in 87% of patients (n = 80), in 13% of cases (n = 12) human papillomavirus was not detected by DNA research; signs of papillomas were noted histologically. Conclusion. Virological studies identified HPV types 6 and 11 among 15 types in 87% of cases in patients with RRP; other types were not detected. In 13% of cases, the virus was not identified. Determination of the level of HPV viral burden revealed a parallel with the clinical course (proliferation, recurrence rate) in children and adults and determined the pattern of digital values. According to the HPV viral burden and clinical data (points on the Dergay scale), it is possible to predict the course of RRP and make recommendations for treatment.
Keywords: Respiratory papillomatosis, Children, Adults, Human papillomavirus, Diagnostics, Viral burden
Cite this paper: Nadjimutdinova N. Sh., Inoyatova F. I., Abdukayumov A. A., Khegay T. R., Development Factors of Recurrent Respiratory Papillomatosis, American Journal of Medicine and Medical Sciences, Vol. 11 No. 12, 2021, pp. 921-925. doi: 10.5923/j.ajmms.20211112.16.
Article Outline
1. Introduction
- Respiratory recurrent papillomatosis (RRP) is a term used to describe an upper respiratory infection caused by human papillomavirus (HPV) [1,4,5]. Although papillomas can be present anywhere along the respiratory tract from the nose to the lungs, they mostly appear in the larynx (over 95% of cases) [1]. RRP manifests itself in both adulthood and early childhood - juvenile-onset respiratory recurrent papillomatosis [4,8]. A national study conducted in the United States from 1994 to 2007 found the proportion of juvenile respiratory papillamatosis of the larynx from 0.24 to 1.11 cases per 100,000 population. More recent studies (2009) showed a wider range of data from 1.98 to 3.21 per 100,000 population [2].The clinical picture of RRP is unpredictable: from spontaneous remission to aggressive progression with spread throughout the respiratory tube from the flesh to the lungs with the death of the patient [11-12]. Patients with RRP usually have non-specific symptoms of respiratory tract lesion, including chronic cough, dysphonia and labored breathing [13-14]. RRP often imitates other diseases such as laryngitis, asthma, stridor, therefore, mistakes in diagnosis and errors in patient treatment often occur [15]. This is especially true of juvenile RRP which is characterized by more aggressive and recurrent course, and therefore is considered separately [9]. Understanding the progressive nature of RRP and its potentially lethal consequences is crucial for effective intervention. Moreover, it is necessary to understand the characteristic symptoms that should cause early suspicion of this condition, despite the relative rarity of the disease [16]. In adults, RRP proceeds less aggressively relative to the frequency of recurrence, but malignancy is typical for adults which is associated with the same factors as the factors of the formations development on the vocal cords [10].Among high-tech diagnostics, the first place is taken by polymerase chain reaction (PCR) - a method of in vitro amplification of a target DNA. At the same time, not only the types of HPV of the main genotypes are determined, but also their quantitative content (viral burden). Quantitative definition of the HPV content provides an interpretation of the prognosis of HPV infection course (the presence of a transient infection, the development of dysplasia or a high risk of its progression) relative to genital HPV [3]. This study was conducted in Uzbekistan, where the level of migration is high and the social level is not very high. According to the data of the republican scientific center of employment and labor protection among migrants, the main part is made up of young people 1-30 years old (52%). The level of education: 7.7% of migrants have high education, 60% - with a secondary specialized education and 2.3% - with incomplete secondary education, which is the basis of the growth factors of RRP in children [17]. Knowledge of the viral type and its viral burden in patients with RRP (or their families) can be important in controlling relapses and preventing RRP development. We hope, this study will allow controlling relapses and raising public awareness of the importance of knowledge about RRP. There is a gradation of the HPV level in the genitals and its comparison with the prognosis of cervical cancer. However, the levels of HPV viral burden in the genitals are not suitable for interpreting the respiratory tract viral burden data for prognosis.Aim of the study was of the study was to determine the possible relationship between the characteristics of the clinic and the definition of the human papillomavirus with its viral burden.
2. Material and Methods
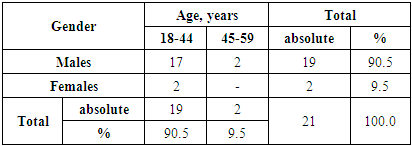
- A study of 92 patients with RRP was carried out. There were 71 children and 21 adults. The criterion for the inclusion of patients in the study was the presence of papillomas in the larynx region, determined by endoscopic examination and confirmed by histological examination. There was no age restriction. The children were at the age from 1 to 17 years (mean age was 4.23 ± 2.1). There were 37 of boys (52.1%) and 34 girls (47.9%). In the age aspect, the group of preschool children (4-6 years) prevailed, have been amounted to 43.7%. The second largest group was young children (1-3 years) - 32.4% of patients. School-age children (7-10 years) made up only 11.3%. (Tab. 1).
|
|
|
3. Methods of Measurement
- Absolute concentration - Copies of HPV DNA in the sample volume (1 ml); Relative concentration - Copies (lg copies) of HPV DNA per cell (genome) - per 1 cell - For 10 ^ 3 cells - For 10 ^ 5 cells.Standard PCR kits of HPV KVANT-21 reagents (DNA Technology, Moscow, Russia) were used to isolate genetic material and to typify/quantify viral DNA. A quantitative study to monitor HPV and identify a possible relationship between the viral burden in a laboratory sample and the clinical course (prevalence, frequency of relapses) was carried out. Method: Polymerase chain reaction with detection of results in real time; qualitative and quantitative multiplex analysis. The principle of the method is based on the use of the process of DNA amplification using the polymerase chain reaction (PCR). The amplification process consists in repeated cycles of temperature denaturation of DNA, annealing of primers with complementary sequences, and subsequent extension of polynucleotide chains from these primers with Taq polymerase.Research using the HPV KVANT reagent kit consists of the stages of DNA extraction (sample preparation) and real-time PCR. To carry out PCR with detection of results in real time we used detecting amplifiers (NPO DNA-Technology LLC): DTlight1, DTprim2 and DT-96 with special software. Detection channels: Fam, Hex, Rox, Cy5. In the kit, an internal control was added to the composition of the mixtures for amplification, designed to assess the efficiency of the polymerase chain reaction. One of the mixtures was intended for amplification of human genomic DNA and is used to control the collection of clinical material and normalize the amount of HPV DNA to the number of human cells in a given sample. List of indicators: Fam - HPV 31, HPV 52 HPV 45 HPV 6, HPV 18, HPV 66, HPV 59; Hex - VK; Rox - HPV 35, HPV 33, HPV 82, HPV 44, HPV 39, HPV 26, HPV 56; Cy5 - HPV 16 HPV 51, HPV 11, HPV 58, HPV 53, HPV 73. The detection limit for HPV genotypes was 5 DNA copies per amplification tube (103 copies / ml of DNA preparation). There are criteria for monitoring the level of genital HPV for predicting cervical cancer: The amount of HPV DNA is not determined in the absence of the virus in the test sample or its minimum amount (below the detectable level) - the risk of developing a pathological process associated with HPV is minimal:• Clinically insignificant concentration of the virus (less than 10 copies of HPV DNA per 103 cells) - the minimum risk of relapse, transient course of the viral process.• A clinically significant concentration of the virus (more than 10 copies of HPV DNA per 103 cells) is a chronic infection with a high risk of developing neoplastic processes.• More than 1000 copies of HPV DNA per 103 cells with the established fact of persistent infection (HPV has been detected for more than 1 year) - increased viral burden.• Reduction of viral burden by 10 times in 6 months - transient infection.• An increase in viral burden 6 months or more after treatment indicates the possibility of relapse.
4. Results
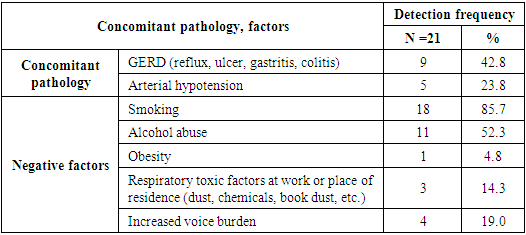
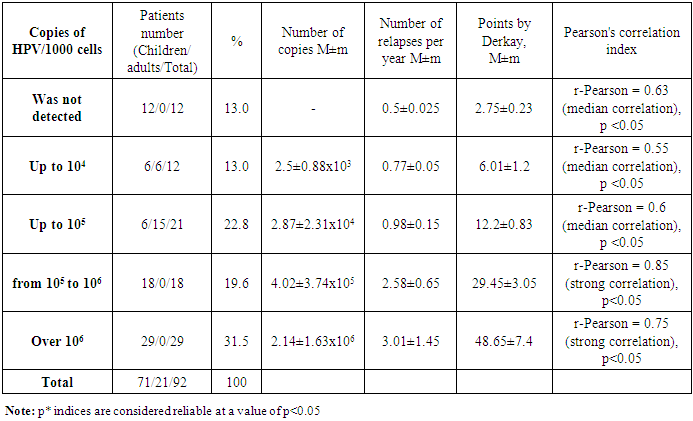
- A study of 92 patients was carried out: typifying revealed only HPV types 6 and 11, other genotypes were not detected. Quantitative analysis revealed the presence of human papillomavirus 6, 11 in 87% of patients (n = 80), in 13% of cases (n = 12) human papillomavirus was not detected by DNA research; signs of papillomas were noted histologically. All patients in whom PCR did not reveal the presence of HPV were children over 7 years old with repeated surgical removal of RRP in early childhood. At the same time, the papillomas remained stable, were not removed for some period after the start of growth. In adults HPV was determined in all cases. We consider that the absence of HPV detection during PCR indicates remission, since clinically, papillomas without growth were observed in this group of patients for a long period. Patients most often admitted for surgical intervention to improve the quality of their voice. In patients in whom HPV was detected, significant discrepancies were noted in the quantitative analysis of viral burden: thus, a larger percentage of patients (31.5%) was detected at the excess of 1,000,000 viral particles (HPV) per 1,000 human cells. However, it is worth noting that among adults, the viral burden did not exceed 100,000 copies of HPV per 1000 cells of the material (Tab. 4).
|
5. Discussion
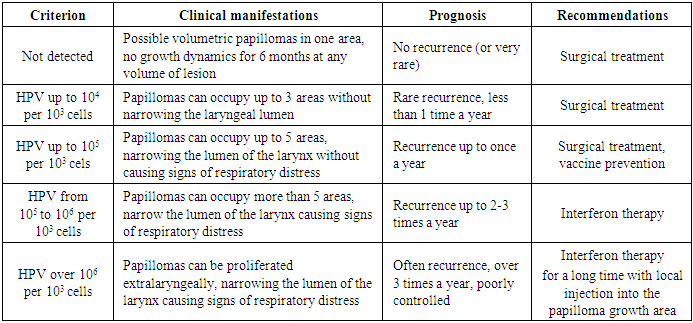
- When comparing the viral burden data and clinical data, it can be stated that at a viral burden up to 10,000 copies per 1000 cells, there was a weak growth of papillomas with rare recurrences up to 1 time per year or less. At the same time, according to the Derkay scale, the proliferation occurs within 2-3 areas (mild course of RRP). With a virus concentration up to 1000 copies or less per 1000 cells, we determined a mild course of RRP with the capture of one or two anatomical regions with rare recurrences associated with previous colds or concomitant viral diseases. More severe clinical course of RRP with frequent recurrences was observed when the virus concentration was more than 1,000,000 copies per 1,000 cells with recurrences up to 3-4 times a year and proliferation of 5-6 areas with a narrowing of II-III degrees. A moderate-severe course with the control of the narrowing degree by surgical methods was observed with a viral burden less than 1,000,000 and higher than 100,000 copies per 1000 cells with the recurrence 2-3 times a year. Patients under constant observation are well controlled, but require special cure (Tab. 5).
|
6. Conclusions
- Virological studies identified HPV types 6 and 11 among 15 types in 87% of cases in patients with RRP; other types were not detected. In 13% of cases, the virus was not identified.Determination of the level of HPV viral burden revealed a parallel with the clinical course (proliferation, recurrence rate) in children and adults and determined the pattern of digital values.According to the HPV viral burden and clinical data (points on the Dergay scale), it is possible to predict the course of RRP and make recommendations for treatment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML